Calcium sulfate hemihydrate/ octacalcium phosphate/ carboxymethyl chitosan composite artificial bone material and preparation method thereof
A technology of carboxymethyl chitosan and calcium sulfate hemihydrate, which is applied in the fields of pharmaceutical formula, medical science, prosthesis, etc., can solve the problems of incomplete degradation, affecting new bone ingrowth, and long time in the body. Achieve the effects of good mechanical strength, good injectability and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] Preparation of calcium sulfate hemihydrate: Using analytically pure calcium sulfate dihydrate as raw material, calcium sulfate hemihydrate is prepared by steam heating method. First, a certain amount of calcium sulfate dihydrate is added to a stainless steel container, and then the corresponding amount has been prepared. A good crystal-transforming agent solution, stirred evenly, reacted in a closed high-temperature and high-pressure reactor under the pressure of 0.15-0.2MPa and 125-135°C, took it out after 4-6 hours, and then put it in and set the temperature at 125-135°C Dry in an oven at ℃, and finally take out, grind and sieve to obtain calcium sulfate hemihydrate powder.
[0030] Preparation of octacalcium phosphate powder
[0031] Prepare calcium acetate solution with a concentration of 0.04M and sodium dihydrogen phosphate solution with a concentration of 0.04M, then slowly add the prepared calcium acetate solution into the sodium dihydrogen phosphate solution in...
Embodiment 1
[0037]Mix 2g of α-calcium sulfate hemihydrate (CSH) with a particle size of less than 100 μm with 0.5 g of octacalcium phosphate and 0.05 g of carboxymethyl chitosan to obtain composite powder A; prepare 0.1 wt % of oxidized Sodium alginate solution, the ratio of oxidized sodium alginate solution to composite powder A is 0.5ml / g.
[0038] When in use, mix the solidified liquid and the composite powder A uniformly for 0.5-1 min, and inject it into the bone defect site.
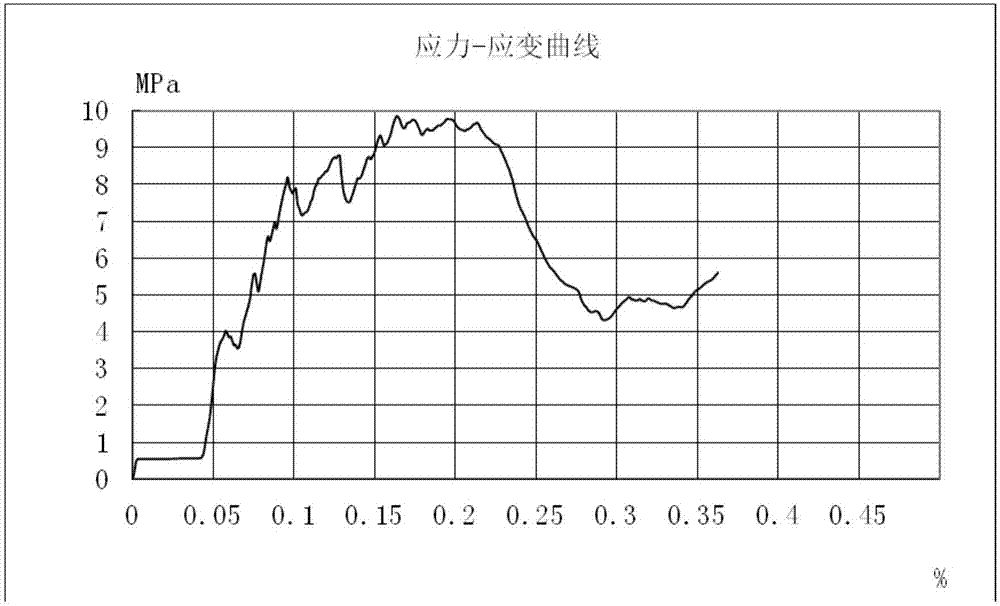
[0039] According to the experiment: the artificial bone material can be initially fixed in 2-3 minutes to prevent its loss, and then the artificial bone material is completely cured in 5-30 minutes. After testing, the compressive strength of the fully cured composite artificial bone material is 4.35Mpa.
[0040] Test experiment of compressive strength of artificial bone material: the composite artificial bone material of calcium sulfate hemihydrate / octacalcium phosphate / carboxymethyl chitosan solidified in a cy...
Embodiment 2
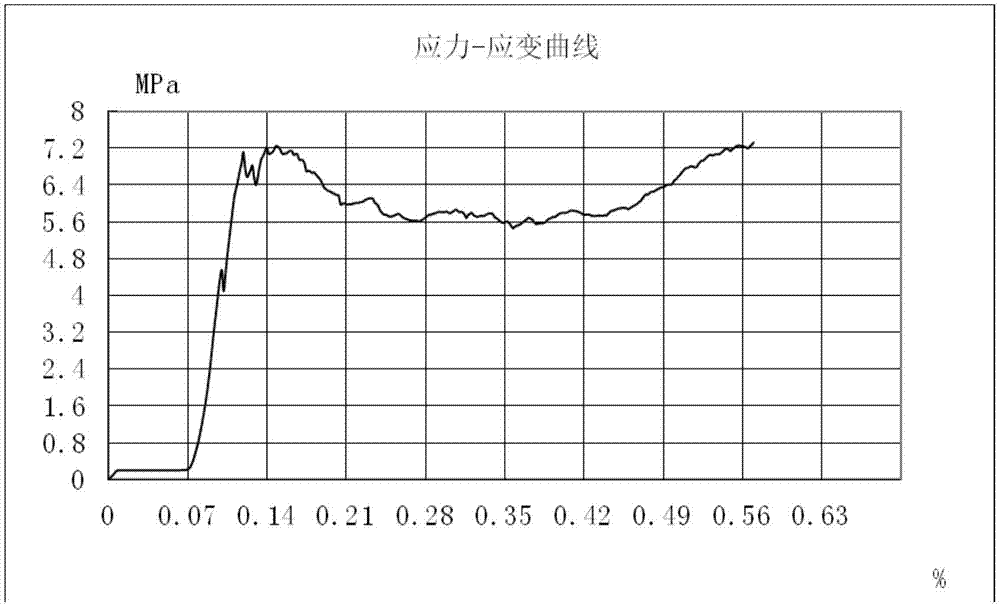
[0042] After mixing 2g of α-calcium sulfate hemihydrate (CSH) with a particle size of less than 80 μm, 1 g of octacalcium phosphate with a particle size of less than 100 μm, and 0.06 g of carboxymethyl chitosan to obtain composite powder A, prepare 0.1 wt % oxidized Konjac solution, the ratio of oxidized konjac solution to composite powder A is 0.4ml / g, to obtain the required artificial bone material. The compressive strength of the final composite artificial bone material test is 6.56Mpa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com