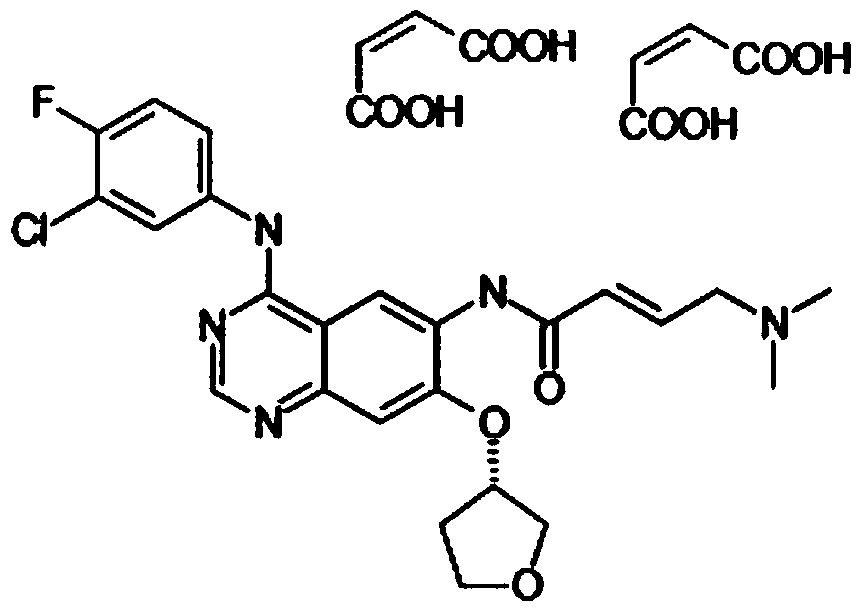
A kind of Afatinib maleate tablet and preparation method thereof
A technology of afatinib and maleic acid, which is applied in the field of medicine, can solve the problems of low hardness, long production cycle, and high friability, and achieve the effects of improving stability, fluidity, and dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] prescription:
[0037]
[0038] Preparation Process:
[0039] ⅰ Dissolve afatinib maleate and menthol in an ethanol solution, cool the solution to -5°C for crystallization under continuous stirring, and obtain complex crystals. Weigh the complex crystals and mix them with mannitol. Add The magnesium stearate is mixed evenly to obtain afatinib maleate tableting mixed powder;
[0040] ⅱThe mixed powder prepared by ⅰ is punched with Ф9mm shallow arc;
[0041] ⅲPut the prepared tablet into an oven for heat treatment at 40°C for 12h;
[0042] iv. The heat-treated tablet of iii is coated with a stomach-dissolving film, and then packaged.
Embodiment 2
[0044] prescription:
[0045]
[0046]
[0047] Preparation Process:
[0048] ⅰ Dissolve afatinib maleate and menthol in ethanol solution, cool the solution to -5°C for crystallization under continuous stirring, and obtain compound crystals. Weigh the compound crystals and mix them with lactose uniformly. Magnesium fatty acid is mixed evenly to obtain afatinib maleate tableting mixed powder;
[0049] ⅱThe mixed powder prepared by ⅰ is punched with Ф9mm shallow arc;
[0050] ⅲPut the prepared tablet into an oven and heat treatment at 50°C for 10h;
[0051] iv. The heat-treated tablet of iii is coated with a stomach-dissolving film, and then packaged.
Embodiment 3
[0053] prescription:
[0054]
[0055] Preparation Process:
[0056]ⅰ Dissolve afatinib maleate and menthol in an ethanol solution, cool the solution to -5°C for crystallization under continuous stirring to obtain a complex crystal, weigh the complex crystal and mix it with sorbitol evenly, add Calcium stearate is mixed evenly to obtain afatinib maleate tableting mixed powder;
[0057] ⅱThe mixed powder prepared by ⅰ is punched with Ф9mm shallow arc;
[0058] ⅲPut the prepared tablet into an oven for heat treatment at 60°C for 8h;
[0059] iv. The heat-treated tablet of iii is coated with a stomach-dissolving film, and then packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com