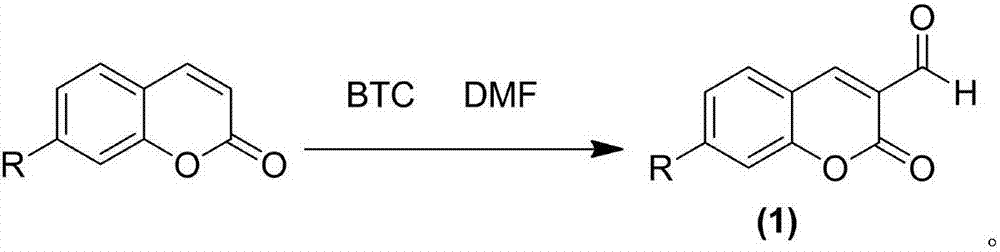
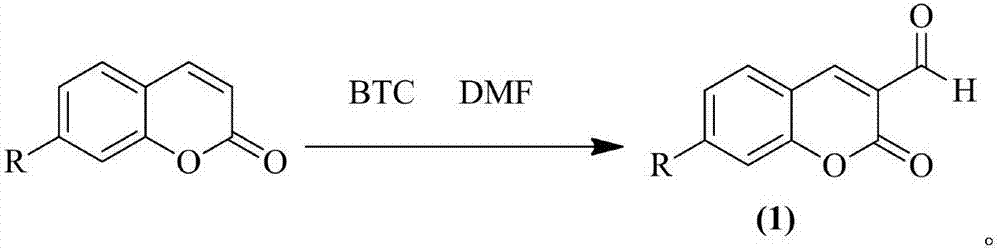
Preparation method of 3-formyl coumarin derivative
A technology of formyl coumarin and coumarin, which is applied in the field of preparation of 3-formyl coumarin derivatives, can solve the problems of dangerous use, transportation and storage, pollute the environment, corrode equipment and the like, and reduce resource consumption. , The reaction process is safe, the effect of small environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The present embodiment provides the synthetic method of 3-formyl-7-diethylamino coumarin, and its main steps are as follows:
[0023] 1.08 g (4.97 mmol) of 7-diethylaminocoumarin was dissolved in 2 mL (26.03 mmol) of DMF, and 1.39 g (4.7 mmol) of BTC was slowly added dropwise under an ice-salt bath when the temperature was kept at -5 °C. A mixed solution of 5 mL of chloroform was added dropwise within 30 min. After all DMF was added dropwise, change to an oil bath, heat, control the temperature to 70°C, monitor the reaction progress by TLC, after 6 hours of reaction, pour directly into 80 mL of water, stir under an ice-salt bath for 10 min, extract with 10 mL of DCM, combine the organic phases under reduced pressure Distillation and ethanol recrystallization to obtain 0.94 g of 3-formyl-7-diethylaminocoumarin orange-red solid with a yield of 76.72%. Melting point 165.4~166.5 ℃.
Embodiment 2
[0025] The present embodiment provides the synthetic method of 3-formyl-7-methoxycoumarin, and its main steps are as follows:
[0026] 0.876 g (4.97 mmol) of 7-methoxycoumarin was dissolved in 2 mL (26.03 mmol) of DMF, and 1.39 g (4.7 mmol) of BTC and 1.39 g (4.7 mmol) of BTC and The mixed solution of 5 mL of chloroform was added dropwise within 30 min. After all DMF was added dropwise, change to an oil bath, heat, control the temperature to 70°C, monitor the reaction progress by TLC, after 6 hours of reaction, pour directly into 80 mL of water, stir under an ice-salt bath for 10 min, extract with 10 mL of DCM, combine the organic phases under reduced pressure Distillation, ethanol recrystallization to obtain 3-formyl-7-methoxycoumarin solid 0.72g, yield 70.12%. The melting point is 235.8~237.5℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com