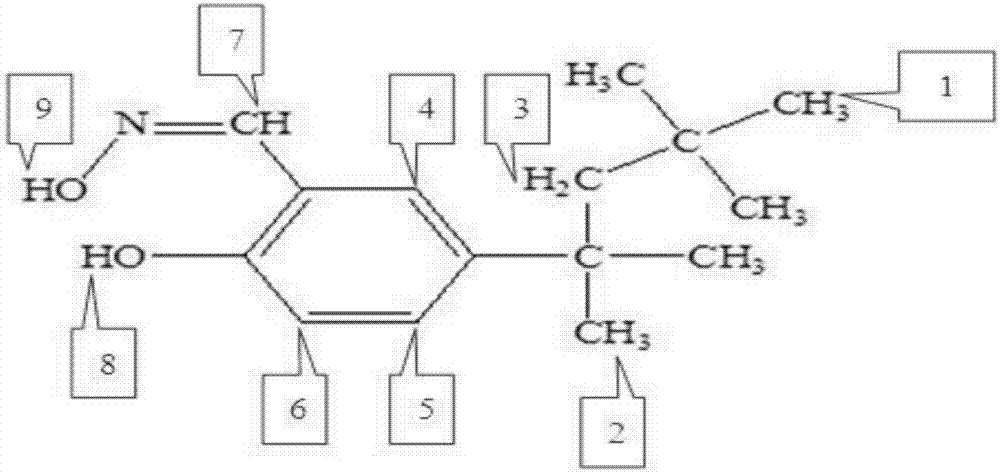
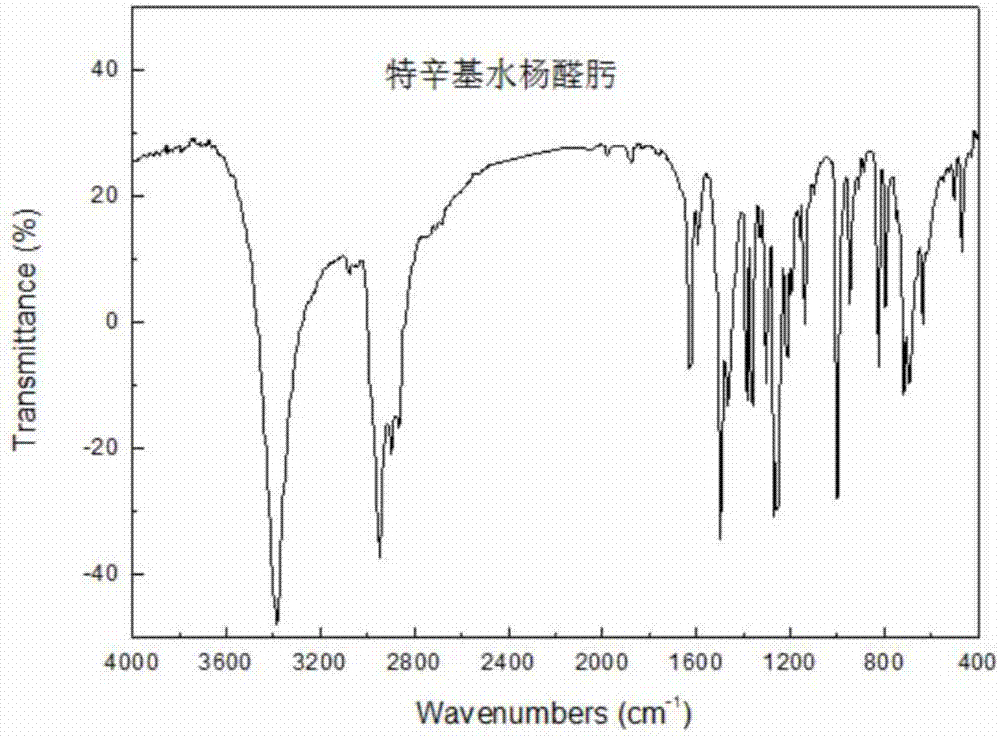
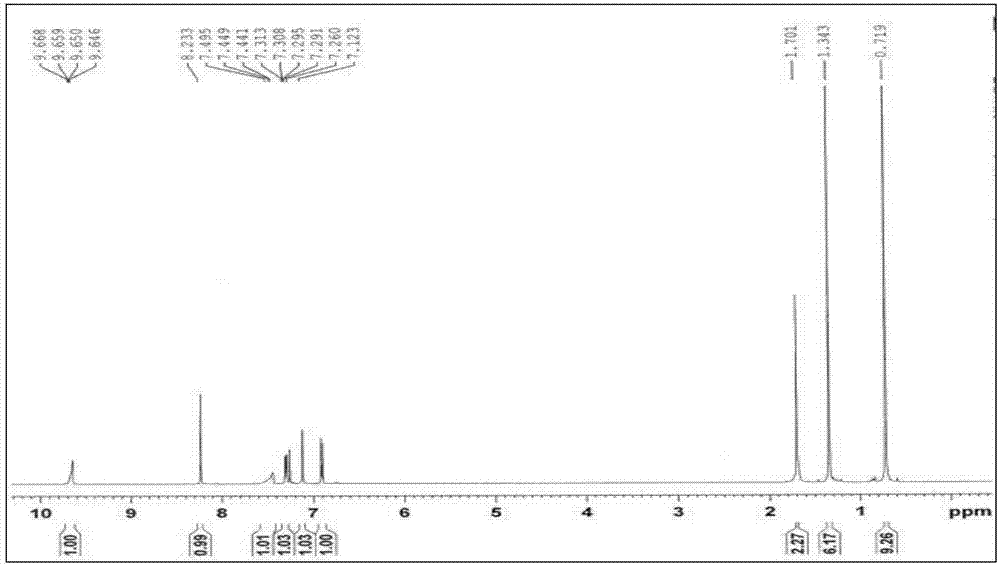
Tert-octyl salicylaldoxime and synthetic method thereof
A technology of tert-octyl salicyl aldoxime and ter-octyl phenol is applied in the field of novel alkyl salicyl aldoxime and its synthesis, and achieves the effects of wide source of raw materials, low synthesis cost and good extraction performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The control synthesis process conditions are as follows: the molar ratio n (magnesium: p-tertoctylphenol) is 0.55:1, the salt-forming reaction time is controlled at 110 minutes, and the salt-forming reaction temperature is controlled at reflux reaction at 63°C; the molar ratio n (more Polyoxymethylene: p-tetraoctylphenol) is 2.40:1, the acylation temperature is controlled at 107° C. with an oil bath, and the acylation reaction time is controlled at 150 minutes; the mol ratio n (hydroxylamine hydrochloride: p-teroctylphenol) is 1.50:1, select the oximation reaction temperature between 45-50°C, control the oil bath temperature at 47°C, and control the oximation reaction time at 210 minutes. Add 2 times of toluene when the methanol-toluene azeotrope is distilled out in the salt formation reaction, and add 5 mL of toluene 3 times during the acylation reaction, with an interval of 2 minutes between each time. After this step, the crude product of tert-octyl salicylaldoxime c...
Embodiment 2
[0022] The control synthesis process condition is as follows: the molar ratio n (magnesium: p-tertoctylphenol) is 0.50: 1, the salt-forming reaction time is controlled at 110 minutes, and the salt-forming reaction temperature is controlled at reflux reaction when 63 ℃; Polyoxymethylene: p-tert-octylphenol) is 2.40: 1, the acylation temperature is controlled at 107 ℃ with an oil bath, and the acylation reaction time is controlled at 150 minutes; the mol ratio n (hydroxylamine hydrochloride: p-tert-octylphenol) is 1.50:1, select the oximation reaction temperature between 45-50°C, control the oil bath temperature at 47°C, and control the oximation reaction time at 210 minutes. Add 2 times of toluene when the methanol-toluene azeotrope is distilled out in the salt formation reaction, and add 5 mL of toluene 3 times during the acylation reaction, with an interval of 2 minutes between each time. After this step, the crude product of tert-octyl salicylaldoxime can be obtained, which ...
Embodiment 3
[0024] The control synthesis process condition is as follows: the molar ratio n (magnesium: p-tertoctylphenol) is 0.50: 1, the salt-forming reaction time is controlled at 90 minutes, and the salt-forming reaction temperature is controlled at reflux reaction when 63 ℃; Polyoxymethylene: p-tert-octylphenol) is 2.40: 1, the acylation temperature is controlled at 107 ℃ with an oil bath, and the acylation reaction time is controlled at 150 minutes; the mol ratio n (hydroxylamine hydrochloride: p-tert-octylphenol) is 1.50:1, select the oximation reaction temperature between 45-50°C, control the oil bath temperature at 47°C, and control the oximation reaction time at 210 minutes. Add 2 times of toluene when the methanol-toluene azeotrope is distilled out in the salt formation reaction, and add 5 mL of toluene 3 times during the acylation reaction, with an interval of 2 minutes between each time. After this step, the crude product of tert-octyl salicylaldoxime can be obtained, which i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com