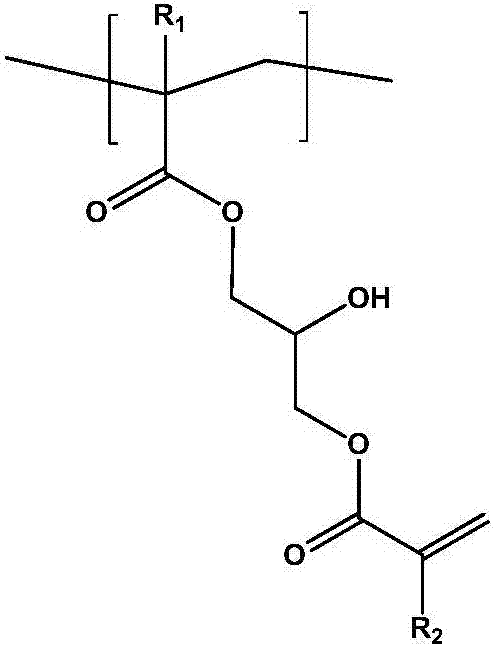
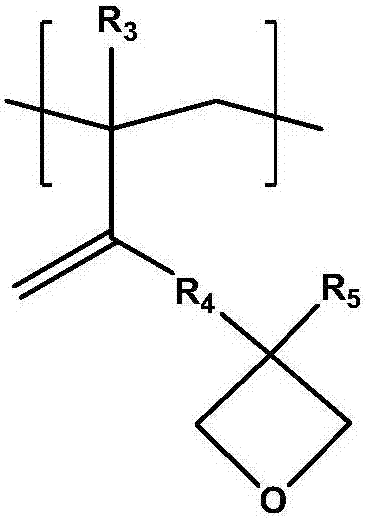
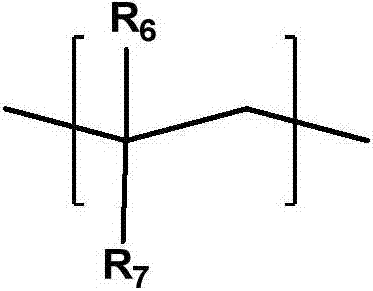
Photosensitive resin composition, photocurable pattern and image display device
A technology of photosensitive resin and composition, which is applied in the field of photosensitive resin composition, can solve the problems of reduced compatibility between photosensitive resin composition and inorganic powder, insufficient solution, and reduced substrate adhesion, etc., to achieve fine Excellent pattern formation, excellent storage stability, and strong pattern effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0130] Synthesis of the first resin A-1-1
[0131] In a 1L flask equipped with a reflux condenser, a dropping funnel, and a stirrer, flow nitrogen at 0.02L / min to form a nitrogen atmosphere, introduce 250 g of propylene glycol monomethyl ether acetate, and heat up to 100°C. In containing acrylic acid 36.0g (0.50 mole), 3-ethyl-3-oxetanyl methacrylate 51.1g (0.30 mole), vinyltoluene 23.6g (0.20 mole) and propylene glycol monomethyl ether acetic acid A solution obtained by adding 3.6 g of 2,2'-azobis(2,4-dimethylvaleronitrile) to a mixture of 150 g of ester was dropped into the flask from the dropping funnel, and stirring was continued at 100°C for 5 hours .
[0132] Next, the atmosphere in the flask was changed from nitrogen to air, and 49.8 g [0.35 mol (70 mol % relative to the acrylic acid used in this reaction)] of glycidyl methacrylate was put into the flask, and the reaction was continued at 110° C. hours, an unsaturated group-containing resin (A-1-1) having a solid con...
Synthetic example 1-2
[0133] Synthesis of First Resin A-1-2
[0134] In a 1L flask equipped with a reflux condenser, a dropping funnel, and a stirrer, flow nitrogen at 0.02L / min to form a nitrogen atmosphere, introduce 250 g of propylene glycol monomethyl ether acetate, and heat up to 100°C. Containing 36.0g (0.50 mole) of acrylic acid, 51.1g (0.30 mole) of 3-ethyl-3-oxetanyl methacrylate, 33.6g (0.20 mole) of cyclohexyl methacrylate and propylene glycol monomethyl A solution obtained by adding 3.6 g of 2,2'-azobis(2,4-dimethylvaleronitrile) to a mixture of 150 g of ether acetate was added dropwise to the flask from the dropping funnel, and further stirring was continued at 100°C 5 hours.
[0135] Next, the atmosphere in the flask was changed from nitrogen to air, and 49.8 g [0.35 mol (70 mol % relative to the acrylic acid used in this reaction)] of glycidyl methacrylate was put into the flask, and the reaction was continued at 110° C. hours, an unsaturated group-containing resin (A-1-2) having ...
Synthetic example 2
[0136] Synthesis of the first resin A-1-3
[0137] In a 1L flask equipped with a reflux condenser, a dropping funnel, and a stirrer, flow nitrogen at 0.02L / min to form a nitrogen atmosphere, introduce 250 g of propylene glycol monomethyl ether acetate, and heat up to 100°C. Containing 36.0g (0.50 mole) of acrylic acid, 51.1g (0.30 mole) of 3-ethyl-3-oxetanyl methacrylate, 20.8g (0.20 mole) of styrene and propylene glycol monomethyl ether acetate The solution obtained by adding 3.6 g of 2,2'-azobis(2,4-dimethylvaleronitrile) to 150 g of the mixture was dropped into the flask from the dropping funnel, and further stirring was continued at 100° C. for 5 hours.
[0138]Next, the atmosphere in the flask was changed from nitrogen to air, and 49.8 g [0.35 mol (70 mol % relative to the acrylic acid used in this reaction)] of glycidyl methacrylate was put into the flask, and the reaction was continued at 110° C. hours, an unsaturated group-containing resin (A-1-3) having a solid cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com