18F-labeled ethinyloestradiol and preparation method and application thereof
A technology of ethinyl estradiol and 18F, which is applied in the field of 18F-labeled ethinyl estradiol and its preparation, can solve the problems of serious adverse reactions and harsh reaction conditions, and achieve simplified labeling steps and purification time, fast reaction rate and good biological activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
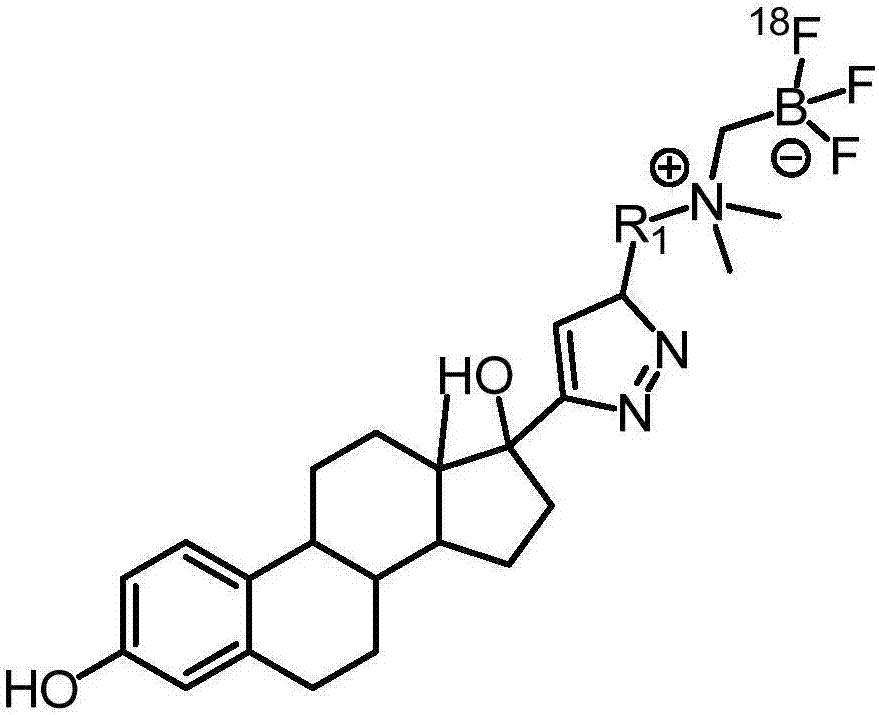
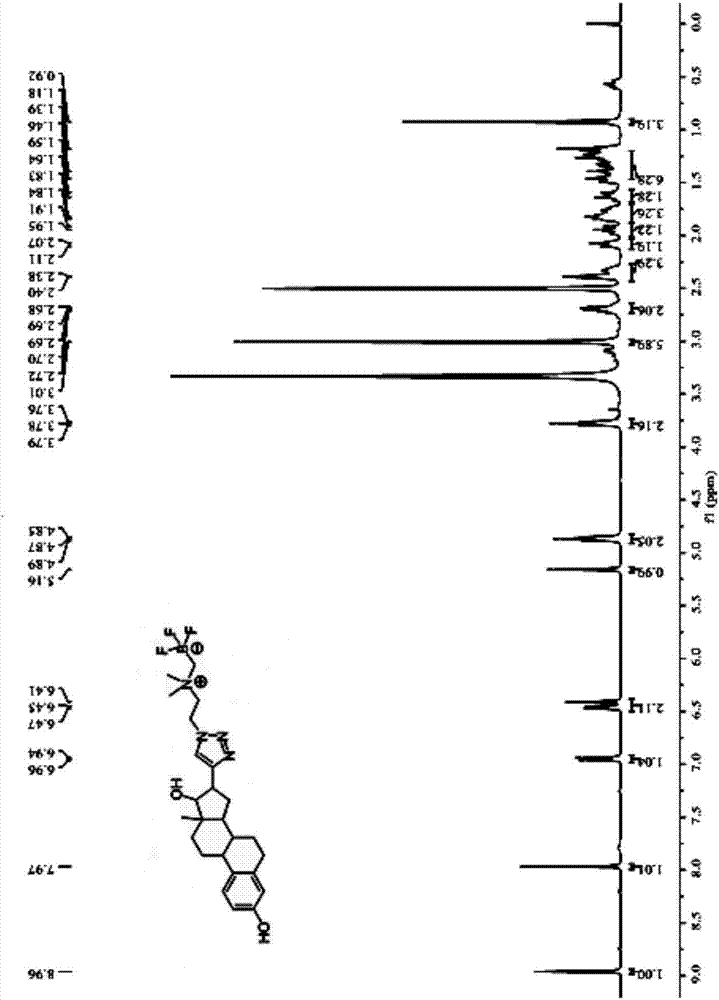
[0076] Described in the present embodiment has formula (I) structure 18 F-labeled ethinyl estradiol, such as figure 1 shown, where R 1 Be ethylene, have formula (I-a) structure:
[0077]
[0078] The synthetic route of compound (I-a) is:
[0079]
[0080] The synthetic steps of the compound shown in above-mentioned formula I-a comprise:
[0081] Q1, mix 2.33g (10mmol) of compound 2-(dimethylamino) bromoethane hydrobromide shown in formula X-1 with 2.60g (40mmol) of sodium azide, add water 50ml to dissolve, nitrogen protection, 90 React at ℃ for 16h, add Na at the end of the reaction 2 CO 3 The resulting reaction solution was adjusted to a neutral pH value, and the above reaction solution was subjected to TLC detection, developed with dichloromethane:methanol=10:1 (v / v), the chromogen was simple iodine, Rf=0.5, and dichloromethane was used Methane extraction, the obtained organic phase was dried over anhydrous sodium sulfate, and spin-dried to obtain an oily liquid....
Embodiment 2
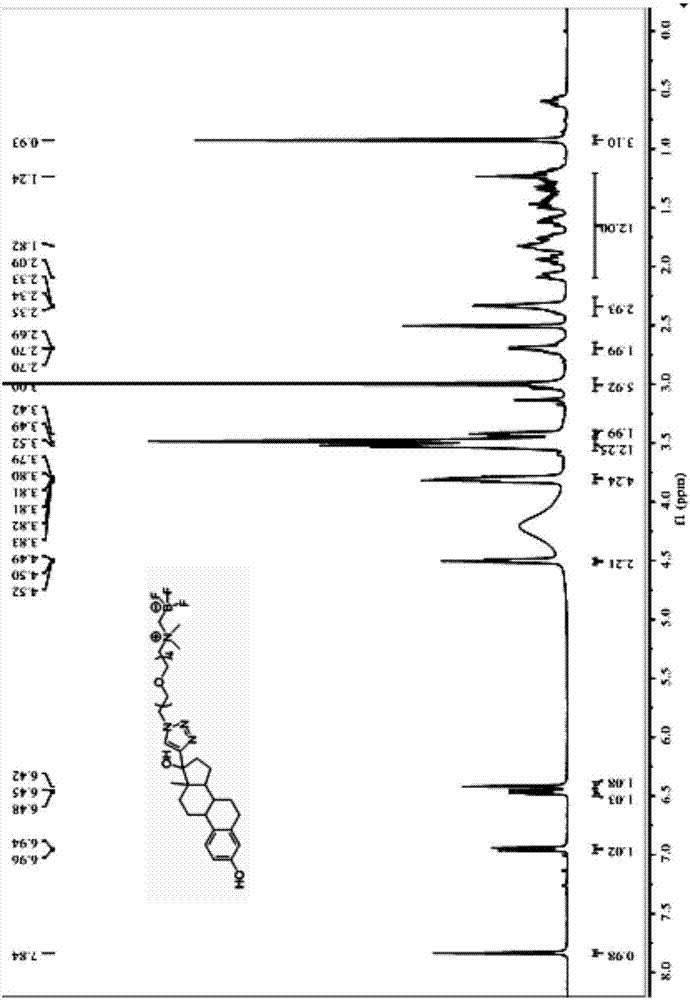
[0088] The structure shown in formula (I) described in the present embodiment 18 F-labeled ethinyl estradiol, such as figure 1 shown, where R 1 Be ethylene, have formula (I-a) structure:
[0089]
[0090] The synthetic route of compound (I-a) is:
[0091]
[0092] The synthetic steps of the compound shown in above-mentioned formula I-a comprise:
[0093] Q1, mix 4.66g (20mmol) of compound 2-(dimethylamino)bromoethane hydrobromide shown in formula X-1 with 4.9g (76mmol) of sodium azide, add water 100ml to dissolve, nitrogen protection, 93 React at ℃ for 15h, add Na at the end of the reaction 2 CO 3 The resulting reaction solution was adjusted to a neutral pH value, and the above reaction solution was subjected to TLC detection, developed with dichloromethane:methanol=10:1 (v / v), the chromogen was simple iodine, Rf=0.5, and dichloromethane was used Methane extraction, the obtained organic phase was dried over anhydrous sodium sulfate, and spin-dried to obtain an oily...
Embodiment 3
[0099] The structure shown in formula (I) described in the present embodiment 18 F-labeled ethinyl estradiol, such as figure 1 shown, where R 1 Be ethylene, have formula (I-a) structure:
[0100]
[0101] The synthetic route of compound (I-a) is:
[0102]
[0103] The synthetic steps of the compound shown in above-mentioned formula I-a comprise:
[0104] Q1. Mix 3493.5mg (15mmol) of compound 2-(dimethylamino)bromoethane hydrobromide shown in formula X-1 with 4095.6mg (63mmol) of sodium azide, add 75ml of water to dissolve, nitrogen protection, 87 React at ℃ for 17h, add Na at the end of the reaction 2 CO 3 The resulting reaction solution was adjusted to a neutral pH value, and the above reaction solution was subjected to TLC detection, developed with dichloromethane:methanol=10:1 (v / v), the chromogen was simple iodine, Rf=0.5, and dichloromethane was used Methane extraction, the obtained organic phase was dried over anhydrous sodium sulfate, and spin-dried to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com