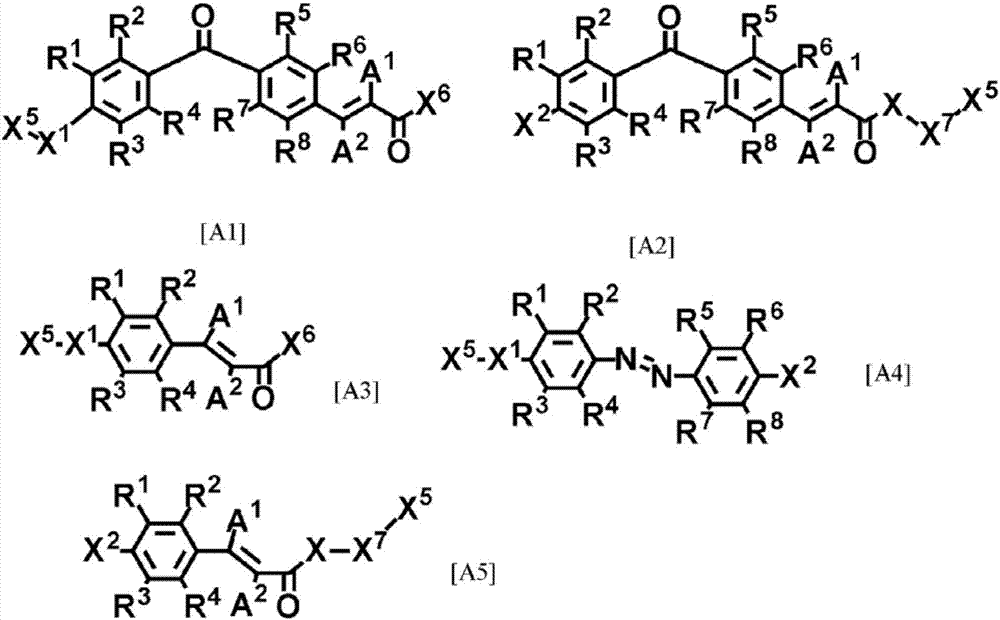
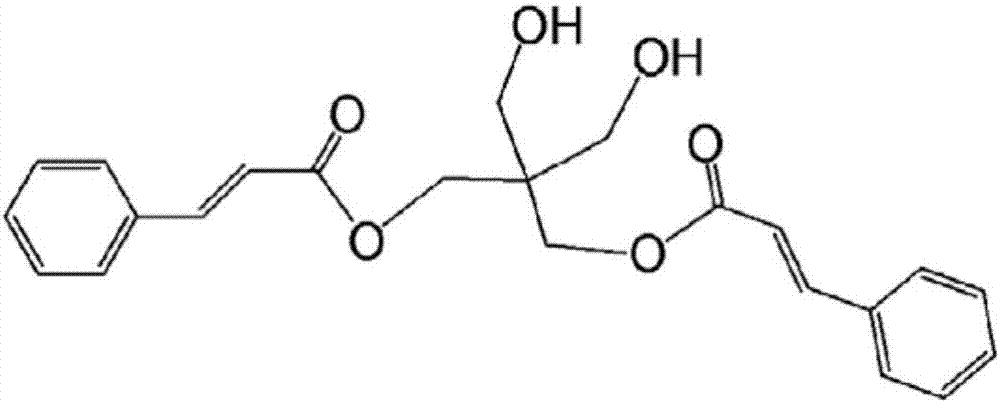
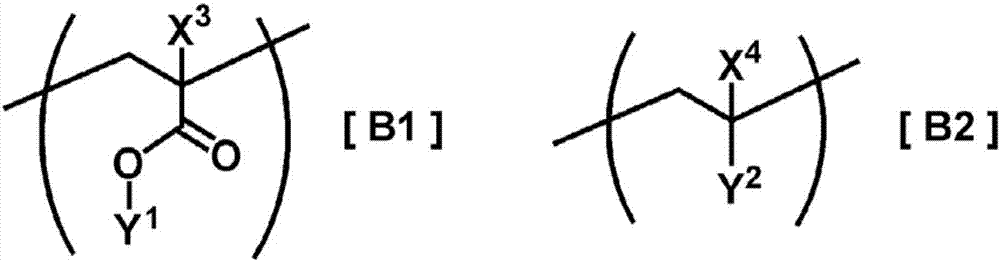
Cured-film formation composition, orientation material, and retardation material
A technology for curing films and compositions, applied in photosensitive materials, instruments, optics and other directions for optomechanical equipment, can solve problems such as recording, and achieve the effects of excellent orientation uniformity and excellent orientation sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0164] Hereinafter, although an Example is given and this embodiment is demonstrated in more detail, this embodiment is not limited to these Examples.
[0165] [Abbreviated symbols used in Examples]
[0166] The meanings of the abbreviations used in the following examples are as follows.
[0167]
[0168] CIN1: Methyl 4-Hydroxyhexyloxycinnamate
[0169] CIN2: Methyl 3-methoxy-4-hydroxyhexyloxycinnamate
[0170]
[0171] MAA: methacrylic acid
[0172] MMA: methyl methacrylate
[0173] HEMA: 2-Hydroxyethyl methacrylate
[0174] AIBN: α,α'-Azobisisobutyronitrile
[0175] PCTO: polycaprolactone triol (molecular weight 500)
[0176] HPCEL: Hydroxypropyl Cellulose
[0177] PHEM: polyhydroxyethyl methacrylate (25% by weight PM solution)
[0178] PEPO: polyester polyol (adipic acid / diethylene glycol copolymer) (molecular weight 4800)
[0179] PCDO: polycarbonate diol (Mw: 500)
[0180]
[0181] HMM: Hexamethoxymethylmelamine
[0182] BMAA: N-(butoxymethyl)acrylamide ...
Synthetic example 1
[0189]2.5 g of MAA, 9.2 g of MMA, 5.0 g of HEMA, and 0.2 g of AIBN as a polymerization catalyst were dissolved in 50.7 g of PM, and reacted at 70° C. for 20 hours to obtain an acrylic copolymer solution (solid content concentration: 25% by mass) (P1). Mn of the obtained acrylic copolymer was 19,600, and Mw was 45,200.
Synthetic example 2
[0191] 25.0 g of BMAA and 1.04 g of AIBN as a polymerization catalyst were dissolved in PM 48.4 g, and reacted at 85° C. for 20 hours to obtain an acrylic copolymer solution (solid content concentration: 35% by mass) (P2). Mn of the obtained acrylic copolymer was 4800, and Mw was 3100.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com