A method for interfacial formation of large-capacity liquid metal batteries
A technology of a liquid metal battery and a chemical formation method, which is applied in the field of electrochemical energy storage, can solve the problems such as the inability to promote the balanced distribution of a large number of metal ions, the difficulty in controlling the rate and distribution uniformity, and the increase in the amount of positive and negative active materials, so as to prevent the interface The effect of enrichment, avoiding short-circuit failure, and improving the quality of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
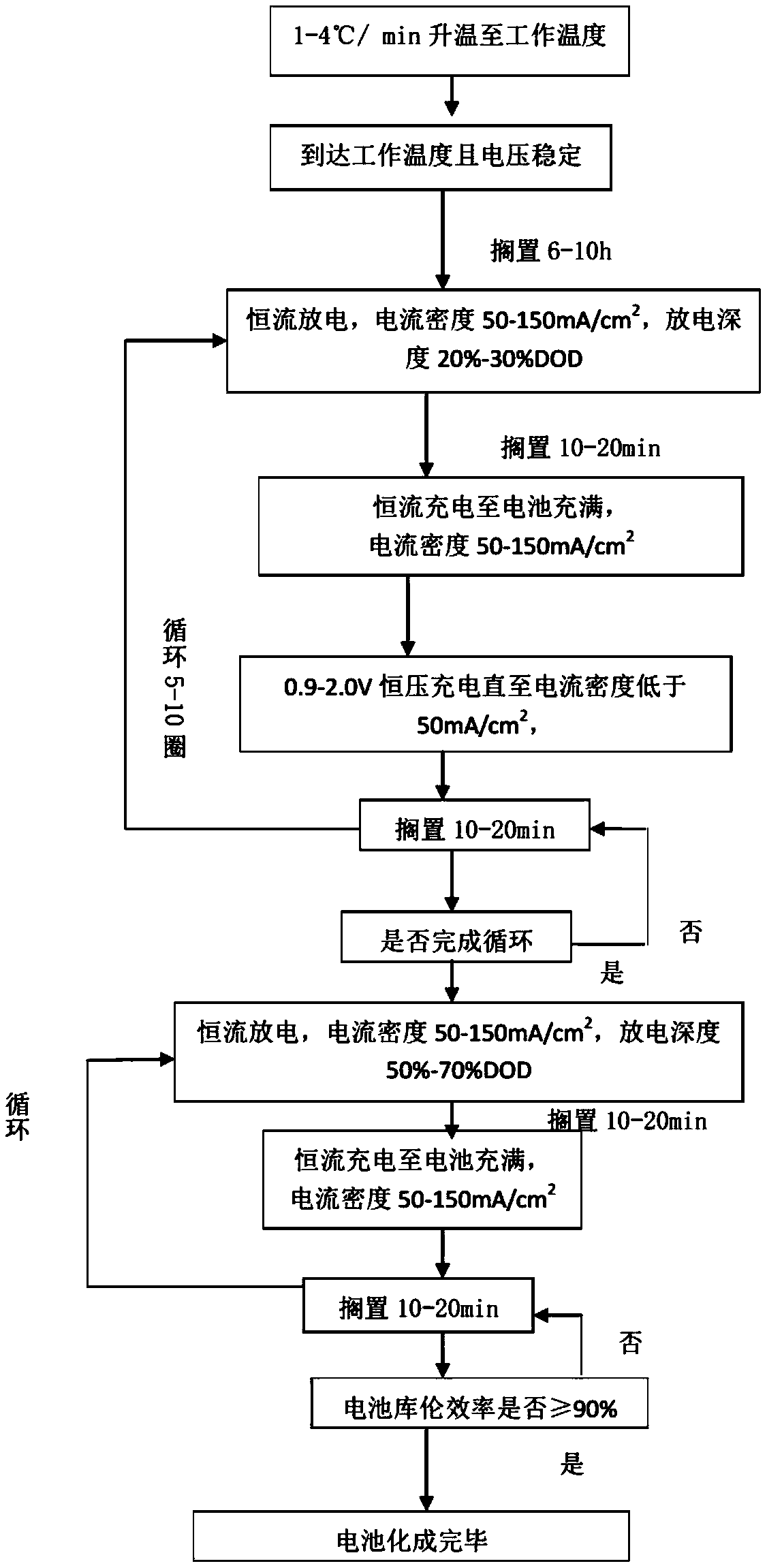
[0039] This example uses a liquid metal battery with a capacity of 100Ah (denoted as 1 # battery), 1 # The interface forming method of the battery mainly includes the following steps:
[0040] (S1) set 1 # The battery is placed in a heating electric furnace or an incubator, and the 1 # The battery is slowly heated up to its operating temperature at a rate of 1°C / min.
[0041] (S2) When 1 #After the temperature of the battery reaches the working temperature, open the battery test program and detect 1 # battery voltage, to be 1 # After the voltage of the battery stabilized, it was left for 7 hours.
[0042] (S3) to 1 # The battery is discharged at a constant current, the discharge current is 6A (the current density is 60mA / cm 2 ), while controlling the discharge time to 300min (5 hours), so that 1 # The depth of discharge of the battery is 30% DOD.
[0043] (S4 will be 1 # After the battery is left for 12 minutes, for 1 # The battery is charged with a constant curren...
example 2
[0051] This example uses a liquid metal battery with a capacity of 150Ah (denoted as 2 # battery), 2 # The interface forming method of the battery mainly includes the following steps:
[0052] (T1) will 2 # The battery is placed in a heating electric furnace or an incubator, and the 2 # The battery is heated up slowly to the working temperature of the battery at a heating rate of 2°C / min.
[0053] (T2) Open the battery test program, check 2 # battery voltage, to be 2 # After the voltage of the battery stabilized, it was left for 8 hours.
[0054] (T3) to 2 # The battery is discharged at a constant current, the discharge current is 18A (the current density is 120mA / cm 2 ), while controlling the discharge time to 125min, so that 2 # The depth of discharge of the battery is 25% DOD.
[0055] (T4) will 2 # After the battery is left for 15 minutes, the 2 # The battery is charged with a constant current until it is fully charged, the charging current is 18A (the current d...
example 3
[0063] This example uses a liquid metal battery with a capacity of 150Ah (denoted as 3 # battery), 3 # The interface forming method of the battery mainly includes the following steps:
[0064] (A1) put 3 # The battery is placed in a heating electric furnace or an incubator, and the 3 # The temperature of the battery is slowly raised to its operating temperature at a rate of 3°C / min.
[0065] (A2) Open the battery test program, check 3 # battery voltage, to be 3 # After the voltage of the battery stabilized, it was left for 9 hours.
[0066] (A3) to 3 # The battery is discharged at a constant current, the discharge current is 7.5A (the current density is 50mA / cm 2 ), while controlling the discharge time to be 240min, so that the battery discharge depth is 20% DOD.
[0067] (A4) put 3 # After the battery has been left for 16 minutes, for 3 # The battery is charged with a constant current until it is fully charged, the charging current is 7.5A (the current density is 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com