A High Performance Liquid Chromatographic Method for Derivative Detection of Steroidal Compounds A-ring Double Bond Isomers
A high-performance liquid chromatography and steroidal compound technology, applied in the field of drug analysis, can solve the problems of inability to achieve complete baseline separation, achieve good promotion and application prospects, good separation effect, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Instrument and chromatographic conditions:
[0060] Shimadzu LC-10AD high performance liquid chromatograph; the chromatographic column is a Techmate C18-ST chromatographic column (250mm×4.6mm×5μm); the column temperature is 30°C; the mobile phase is water-acetonitrile (55:45); The chromatographic liquid phase system is used for detection, the injection volume is 10 μL; the flow rate is 1.3ml / min; an ultraviolet detector is used; the detection wavelength is 200nm.
[0061] Experimental procedure
[0062] Derivation: Accurately weigh an appropriate amount of the sample and place it in a 25ml round bottom flask, accurately add an appropriate amount of dichloromethane to dissolve it, and then add m-chloroperoxybenzoic acid at a molar ratio of 1:1 to the sample. After reacting in a water bath at 25°C for 1 hour, remove the dichloromethane, and then accurately add an appropriate amount of acetonitrile to dissolve it, transfer it out, and obtain the product.
[0063] Prepara...
Embodiment 2
[0070] Instrument and chromatographic conditions:
[0071] Shimadzu LC-10AD high-performance liquid chromatography; the chromatographic column is Techmate C18-ST chromatographic column (250mm×4.6mm×5μm); the column temperature is 30°C; water-acetonitrile (55:45) is used as the mobile phase; Detection is performed with a high-performance chromatographic liquid phase system. The injection volume is 10 μL; the flow rate is 1.5 ml / min; an ultraviolet detector is used; the detection wavelength is 200 nm.
[0072] All the other operating steps and conditions are the same as in Example 1.
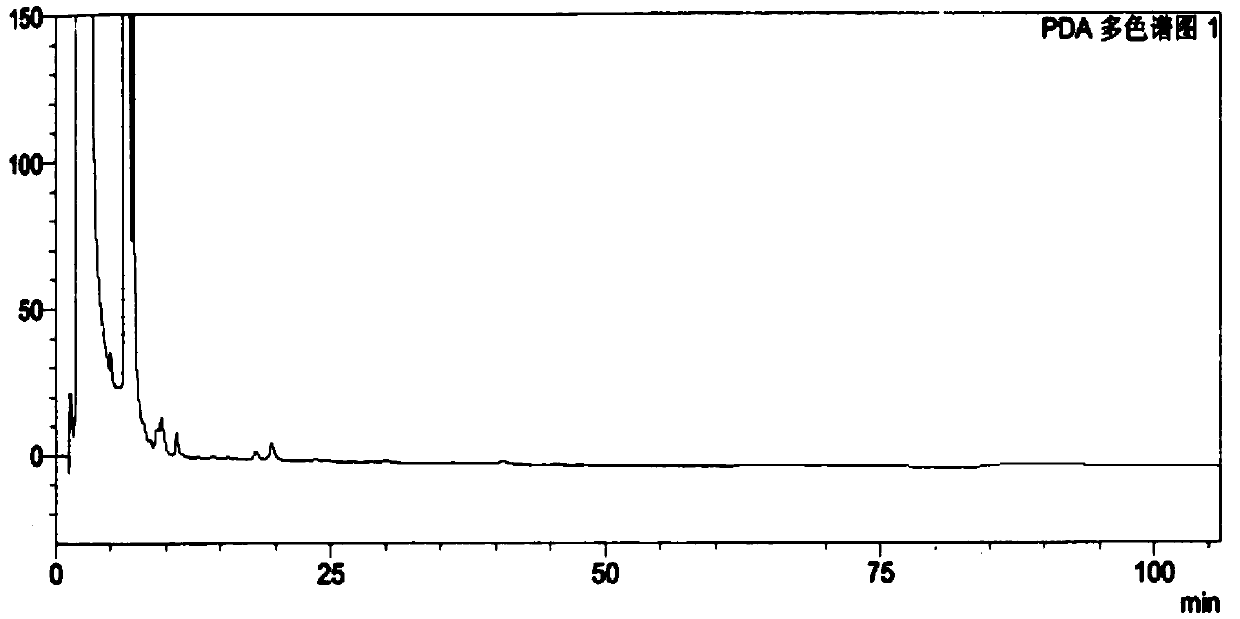
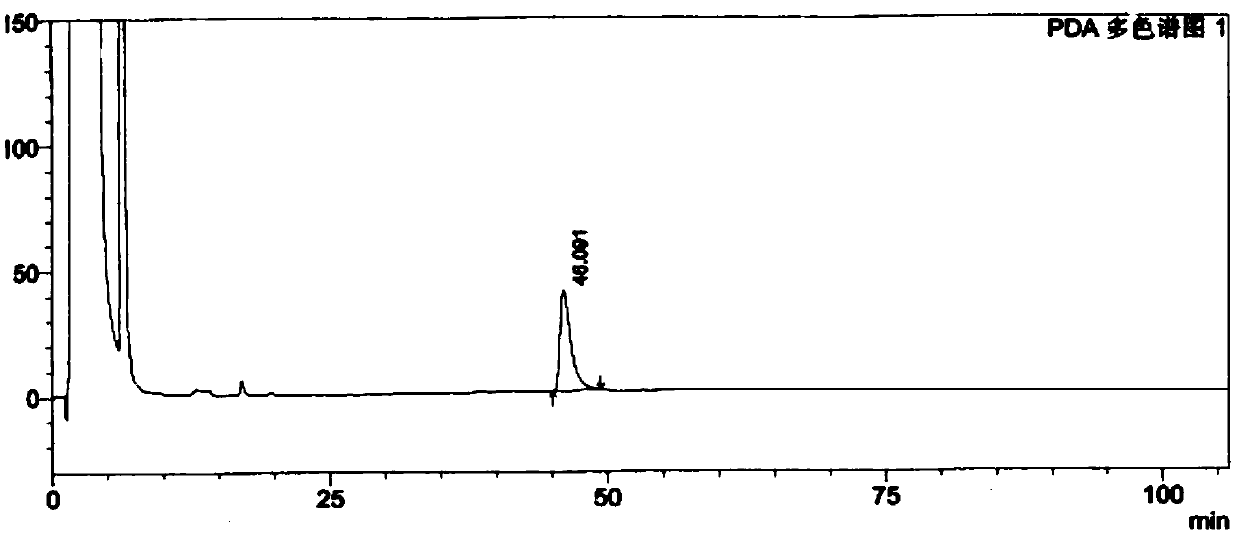
[0073] The test results show that the solvent peak has no interference to the isomers, and the peaks of the resolution solution isomers can be completely separated. See Figure 5 .
Embodiment 3
[0075] Instrument and chromatographic conditions:
[0076] Shimadzu LC-10AD high performance liquid chromatograph; the chromatographic column is a Techmate C18-ST chromatographic column (250mm×4.6mm×5μm); the column temperature is 35°C; the mobile phase is water-acetonitrile (55:45); Chromatographic liquid phase system for detection The injection volume is 10 μL; the flow rate is 1.3ml / min; an ultraviolet detector is used; the detection wavelength is 200nm.
[0077] All the other operating steps and conditions are the same as in Example 1.
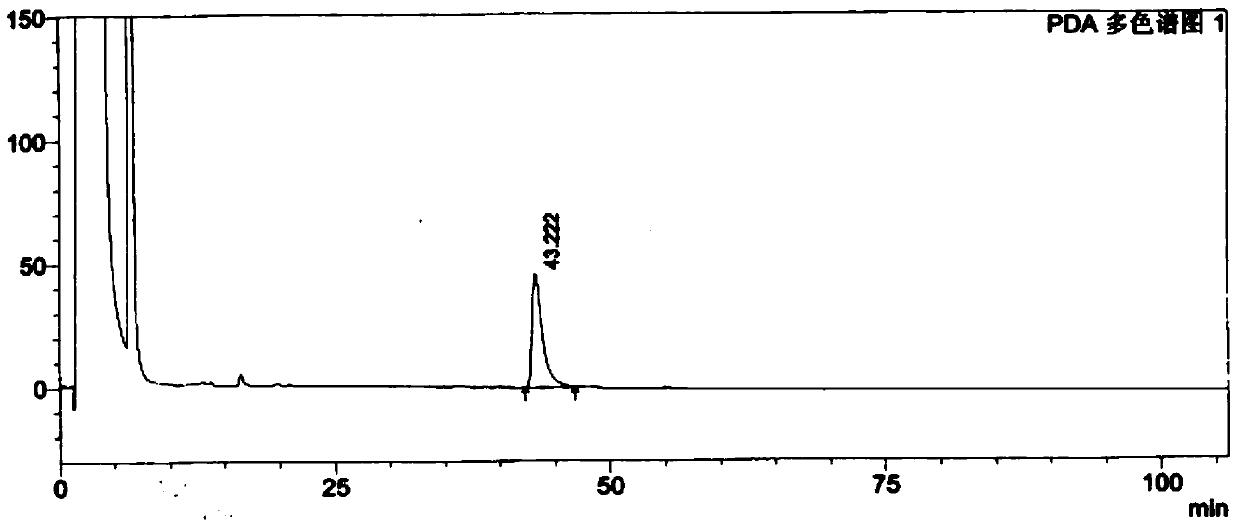
[0078] The test results show that the solvent peak has no interference on the isomers, and the peaks of the resolution solution isomers can be completely separated, see Figure 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com