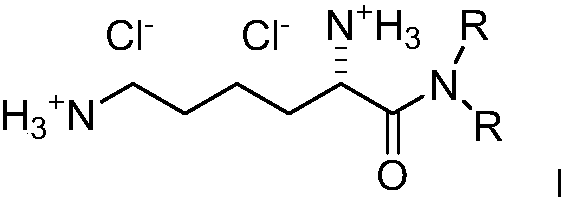
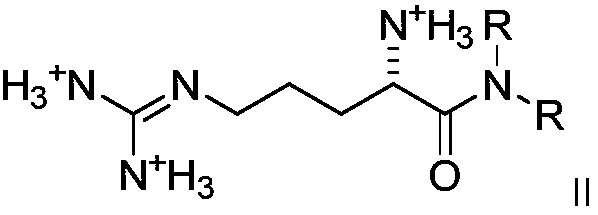
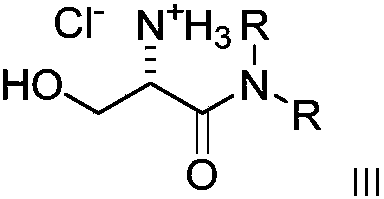
Dialkyl cationic amphiphilic antibacterial peptide mimetic with antibacterial activity and preparation method thereof
A dialkyl cationic antibacterial activity technology, applied in the field of dialkyl cationic amphiphilic antibacterial peptide mimics and their preparation, to achieve significant selectivity, broad-spectrum antibacterial activity, and good antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 compound 4a-4r
[0063] (1) Synthesis of compounds (1d, 1f, 1h):
[0064] Compounds (1a-1c, 1e, 1g) are commercially available. Compounds (1d, 1f, 1h) were synthesized by the traditional method: add solvent DMF (N,N-dimethylformamide) to a flask containing a mixture of n-alkylamine and brominated alkanes, and then add anhydrous carbonic acid Potassium (n-alkylamine: bromoalkane: anhydrous potassium carbonate = 1:1:1), then stirred at 80°C for 12h. Add chloroform and water after the reaction to extract, separate the organic layer, extract three times with chloroform, wash twice with water, wash once with saturated brine, dry over anhydrous magnesium sulfate, filter and evaporate to dryness, and the crude product is separated by column chromatography (petroleum ether : ethyl acetate: triethylamine=10:1:0.05).
[0065] (2) Synthesis of compound (2a-2i):
[0066] Synthesis of compound (2a, 2e-2i): 5g of amino acids (L-lysine, L-alanine, L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com