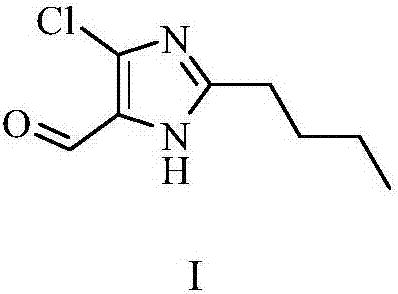
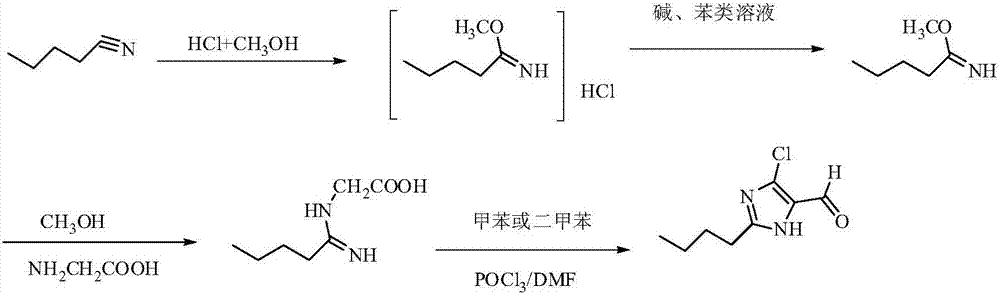
Preparation method of 2-n-butyl-4-chloro-5-formylimidazole
A technology of formyl imidazole and n-butyl, applied in the field of preparation of losartan intermediate 2-n-butyl-4-chloro-5-formyl imidazole, can solve high production cost, poor product quality, difficult to purchase To achieve the effect of low production cost, less impurity generation and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
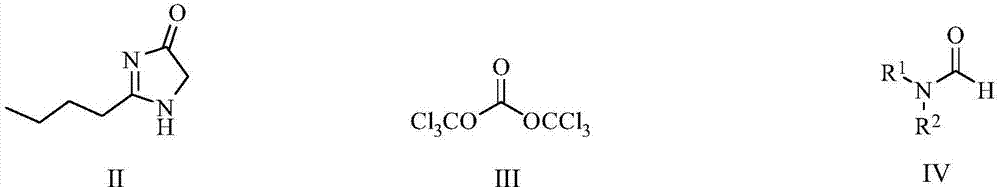
[0041] The preparation of embodiment 1 (1-iminopentyl) glycine tert-butyl ester
[0042]
[0043] Add pentamidine hydrochloride (1.36kg, 10mol) and glycine tert-butyl ester hydrochloride (1.67kg, 10mol) into DMF (10L), add triethylamine (3.54kg, 35mol) dropwise, and heat up to 60°C , stirred for 8 hours, cooled to room temperature, added water and ethyl acetate, separated the organic phase, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated to dryness to give (1-iminopentyl) glycine tert-butyl ester, 1.35kg , Yield: 86.1%.
Embodiment 2
[0044] The preparation of embodiment 2 (1-iminopentyl) glycine tert-butyl ester
[0045] Add pentamidine hydrochloride (1.36kg, 10mol) and glycine tert-butyl ester hydrochloride (2.51kg, 15mol) into DMF (20L), add triethylamine (4.05kg, 40mol) dropwise, and heat up to 60°C , stirred for 10 hours, cooled to room temperature, added water and ethyl acetate, separated the organic phase, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated to dryness to obtain (1-iminopentyl) glycine tert-butyl ester, 1.38kg , Yield: 86.7%.
Embodiment 3
[0046] Example 3 Preparation of 2-n-butyl-4-chloro-5-formyl imidazole
[0047]
[0048] (1-iminopentyl)glycine tert-butyl ester (1.35kg, 6.3mol) was added to 20L of 10% trifluoroacetic acid 1,2-dichloroethane solution, stirred at room temperature for 24 hours, the reaction was completed, evaporated to dryness , add toluene 15L, phosphorus oxychloride (9.64kg, 63mol) warm up to 80°C, stir for 2 hours, add DMF 4.6kg dropwise, heat up to 105°C, stir for 7 hours, the reaction is complete, cool down to 0°C, pour hydrogen Add ethyl acetate to the aqueous solution of sodium oxide, separate the layers, dry the organic layer over anhydrous sodium sulfate, evaporate to dryness, and recrystallize with ethyl acetate n-heptane to obtain 2-n-butyl-4-chloro-5-formyl imidazole , 1.05kg, purity 99.5%, yield 89.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com