Injectable self-sealing gel applicable to drug loading and releasing and preparation method and application thereof
An injection-type, self-healing technology, applied in pharmaceutical formulation, drug delivery, medical science, etc., can solve problems such as weak mechanical strength, inability to accurately control drug release, difficulty in taking into account biocompatibility and biodegradability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
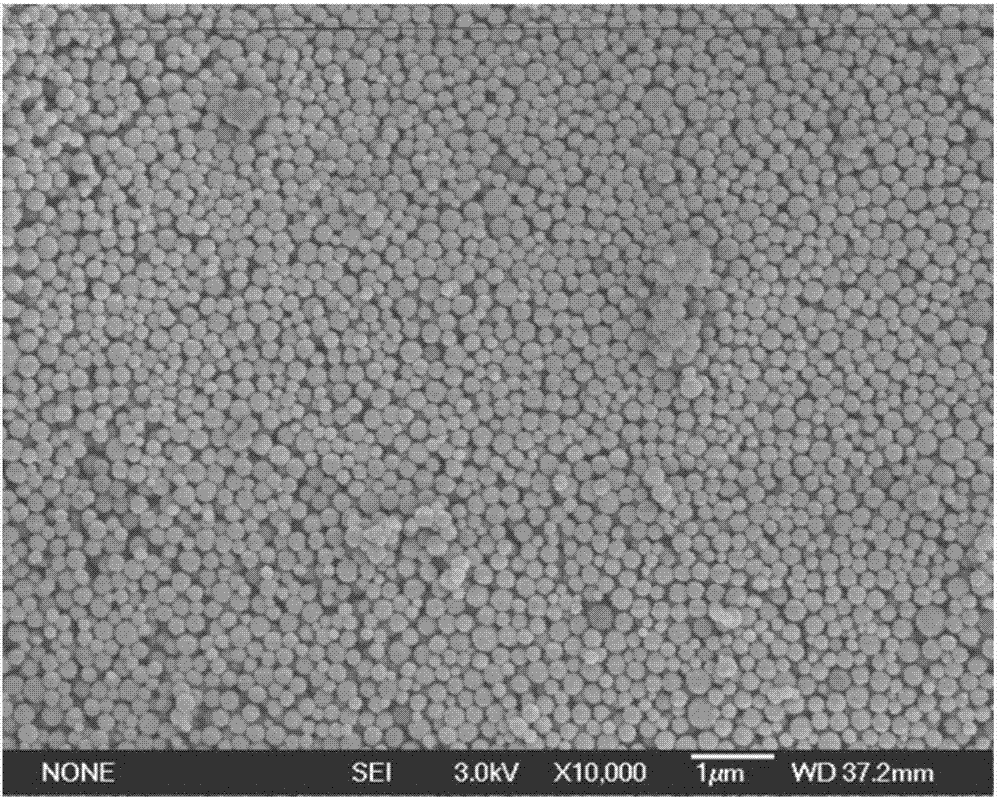
[0047] Using type A gelatin as raw material, heat it in deionized water at 40°C to dissolve it, prepare a gelatin aqueous solution with a concentration of 5w / v%, adjust the pH value of the gelatin aqueous solution to 3, and then add acetone 3 times the volume of the solution to the solution to generate The dispersion of gelatin microgel particles; adding different amounts of 25wt% glutaraldehyde aqueous solution to the dispersion respectively to crosslink the gelatin microgel particles, the amount of glutaraldehyde added is respectively 25wt% glutaraldehyde per gram of gelatin Aldehyde 66μL, 132μL, 264μL, 538μL, cross-linking reaction time 12hr, then add glycine to neutralize the unreacted aldehyde group, centrifuge and wash to obtain the dispersion liquid of type A gelatin microgel particles.
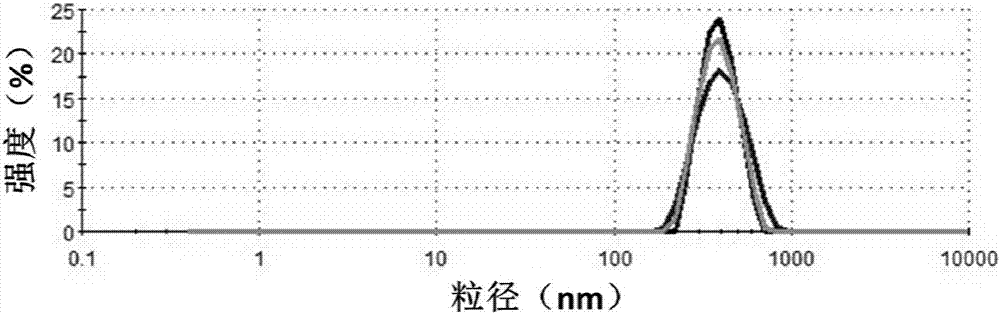
[0048] The particle size analysis of type A gelatin particles prepared with different crosslinking agent dosages was carried out using a laser particle size analyzer, and the results ar...
Embodiment 2
[0054]Use type A gelatin and type B gelatin as raw materials respectively, dissolve them in deionized water and heat at 40°C, and prepare an aqueous solution of type A gelatin with a concentration of 5w / v% and type B gelatin with a concentration of 5w / v% aqueous solution, adjust the pH value of the two gelatin aqueous solutions to be 3, and then add 3 times the volume of acetone to the solution to generate type A gelatin and type B gelatin microgel particle suspensions; add 25wt% pentadiene to the suspension respectively Aldehyde aqueous solution, to cross-link the gelatin microgel particles, the amount of glutaraldehyde added is 66 μL of 25wt% glutaraldehyde per g gelatin, the cross-linking reaction time is 12hr, then add glycine to neutralize the unreacted aldehyde groups, centrifuge and wash separately A type A gelatin particle dispersion and a type B gelatin particle dispersion were obtained. The particle size and zeta potential data of the prepared microgel measured by la...
Embodiment 3
[0067] Use type A gelatin as the raw material, dissolve it under heating at 40°C, prepare a type A gelatin aqueous solution with a concentration of 5w / v%, adjust the pH value to 11, and then add 3.5 times the volume of ethanol to the solution to generate type A gelatin Microgel particle suspension; add 25wt% glutaraldehyde aqueous solution to the suspension to cross-link the gelatin microgel particles. The amount of glutaraldehyde added is 66 μL of 25wt% glutaraldehyde per gram of gelatin. After 12 hours, type A gelatin microgel particles were obtained, and the particle size and surface zeta point parameters are shown in Table 5.
[0068] Prepare alginate microgel particles by emulsion method, the specific preparation method is as follows: add 1wt% sodium alginate aqueous solution into calcium chloride aqueous solution and continue high-speed stirring (stirring speed>5000rpm), that is, calcium alginate particles, particles The size and surface zeta point parameters are shown i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com