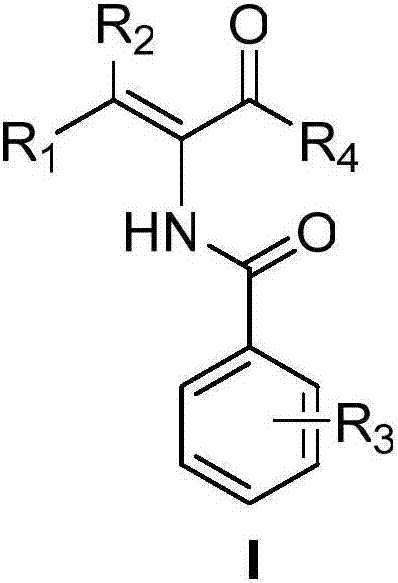
Acrylamide compound and preparation method and medical application thereof
A compound and propylamine-based technology, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve problems such as inability to completely eliminate viruses, low sustained response rate of interferon, and drugs that cannot meet the needs of treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080](S,E)-N-{1-bromo-3-[(1-hydroxy-3-phenyl)-2-propylamino]-3-oxo-1-phenylpropen-2-yl}benzyl Amide (I 1 )
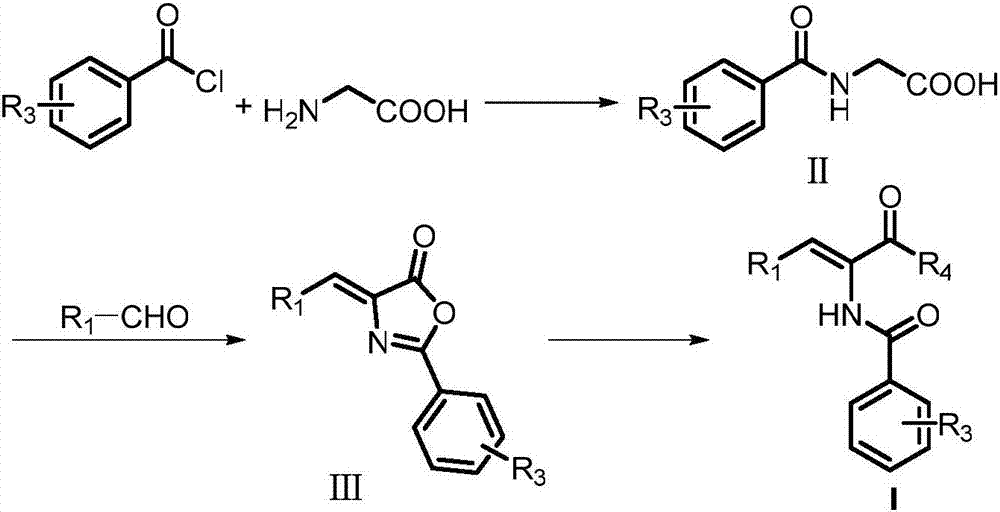
[0081] Glycine (0.75g, 0.01mol) was dissolved in sodium hydroxide (1.2g, 0.03mol) solution, cooled to 0°C in an ice-water bath, benzoyl chloride (2.34mL, 0.02mol) was added dropwise, and after stirring for half an hour, the reaction After the solution was returned to room temperature, hydrochloric acid solution was added until a large amount of white solids were precipitated, the pH was adjusted to about 2, the solids were filtered out, the solids were washed with water for several times, and then dried into white powder solid benzoylglycine (1.492g, 83%). Add acetic anhydride (1.5mL) to three mixtures of benzoylglycine (0.537g, 3mmol), anhydrous sodium acetate (0.246g, 3mmol), and benzaldehyde (0.306mL, 3mmol). Condensate under reflux for 3 hours, extract with dichloromethane after cooling, wash the organic layer with saturated brine and saturated sodium bicarbonate...
Embodiment 2
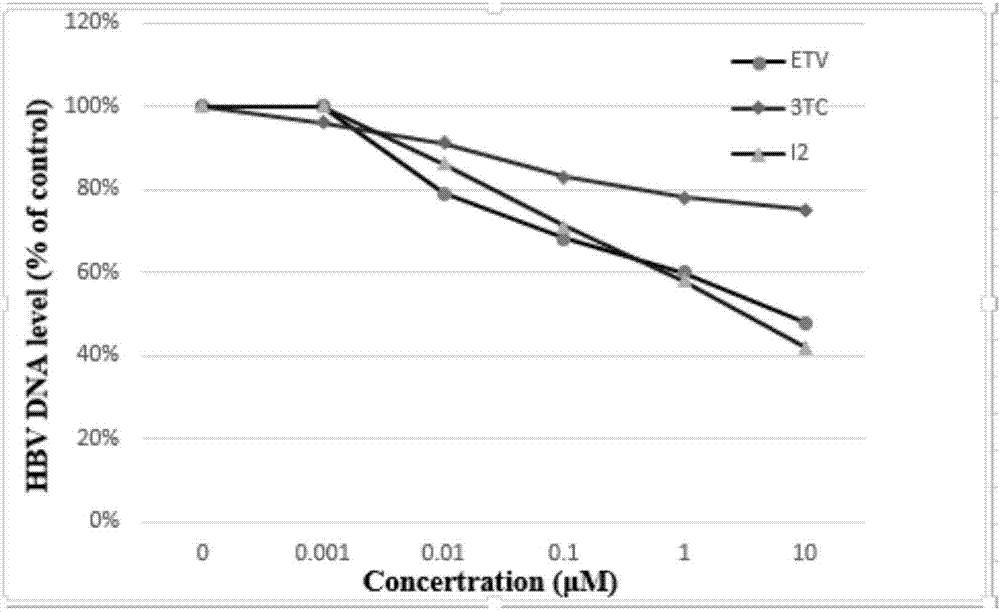
[0086] (S,E)-N-{1-bromo-3-[(1-hydroxy-3-phenyl)-2-propylamino]-1-(4-hydroxyphenyl)-3-oxo-1- Propylene-2-yl}benzamide (I 2 )
[0087] According to the similar method of Example 1, use benzoylglycine (2.02g, 11.3mmol), p-hydroxybenzaldehyde (1.38g, 11.3mmol) to react to obtain (Z)-4-(4-hydroxybenzylidene)-2 -Phenyloxazol-5(4H)-one (2.358g, 78.7%); (Z)-4-(4-hydroxybenzylidene)-2-phenyloxazol-5(4H)-one (3.684 g, 13.9mmol) and L-phenylalaninol (2.1g, 13.9mmol) were reacted for 20h, and an appropriate amount of bromine solution was added dropwise to obtain the yellow title compound (3.6g, 18%).
[0088] m.p.220℃-224℃;
[0089] ESI-MS: m / z 495[M+H] + ;
[0090] 1H-NMR (DMSO, 400MHz, δppm): 2.80-2.96(m, 2H, CH2), 3.41-3.52(m, 2H, CH2), 4.11-4.26(m, 1H, CH), 6.81-6.86(d, 2H,J=8.8Hz,Ar-H),7.10-7.16(m,1H,Ar-H),7.19-7.30(m,4H,Ar-H),7.53-7.59(m,3H,Ar-H) ,7.93-7.98(d,1H,J=8.8Hz,Ar-H),8.04-8.09(m,1H,Ar-H),8.13-8.17(m,2H,Ar-H),8.59-8.60(s ,1H,NH),9.93-10.04(s,1H,OH),10.51-10.55(s,1H,N...
Embodiment 3
[0092] (S,Z)-2-fluoro-N-{3-[(1-hydroxy-3-phenyl)-2-propylamino]-3-oxo-1-phenylpropen-2-yl}benzyl Amide (I 3 )
[0093] Glycine (3.75g, 0.05mol) was dissolved in sodium hydroxide (6g, 0.15mol) solution, cooled to 0°C in an ice-water bath, o-fluorobenzoyl chloride (6mL, 0.05mol) was added dropwise, stirred for 1 hour, and the reaction After the solution returns to room temperature, add hydrochloric acid solution until a large amount of white solids are precipitated, adjust the pH to about 2, filter the precipitated solids, wash the solids with water several times, and dry them into white powder solid o-fluorobenzoylglycine (2.64g, 27% ). Add 1 mL of acetic anhydride to three mixtures of o-fluorobenzoylglycine (1.93 g, 0.01 mol), anhydrous sodium acetate (0.82 g, 0.01 mol), and benzaldehyde (1.02 mL, 0.01 mol), Condensate in the bath and reflux for 4 hours, extract with dichloromethane, wash the organic layer with saturated brine and saturated sodium bicarbonate solution, dry ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com