Method for building BCRP (breast cancer resistance proteins) mediated medicine transport models for research on 3D (three-dimensional) organs of small intestines and application
A construction method and organoid technology, which can be applied in the fields of biochemical equipment and methods, artificial cell constructs, and microbial assay/inspection. and physiological functions, etc., to achieve the effect of efficient, fast, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Isolation of mouse small intestinal crypts and culture of small intestinal 3D organoids
[0075] (1) After 6-8 week old C57BL / 6 mice were killed by cervical dislocation, the small intestine was taken out and placed in pre-cooled PBS.
[0076] (2) After carefully removing the mesenteric adipose tissue with tweezers, cut it from the middle, wash the intestinal contents with pre-cooled PBS, transfer to a sterile 50mL centrifuge tube containing pre-cooled PBS, and place it on ice.
[0077] (3) In a biological safety cabinet, wash 3-4 times with pre-cooled PBS containing penicillin / streptomycin (P / S), transfer the small intestine to 25mL PBS containing 2mM EDTA, and digest in a refrigerator at 4°C for 25 minutes .
[0078] (4) Transfer the digested small intestine to a 50mL centrifuge tube containing 25mL pre-cooled PBS, shake for about 50 times, and collect the suspension.
[0079] (5) Repeat the previous step, filter the suspension into a 50 mL centrifuge tube ...
Embodiment 2
[0084] Example 2 Morphological observation of small intestine 3D organoids
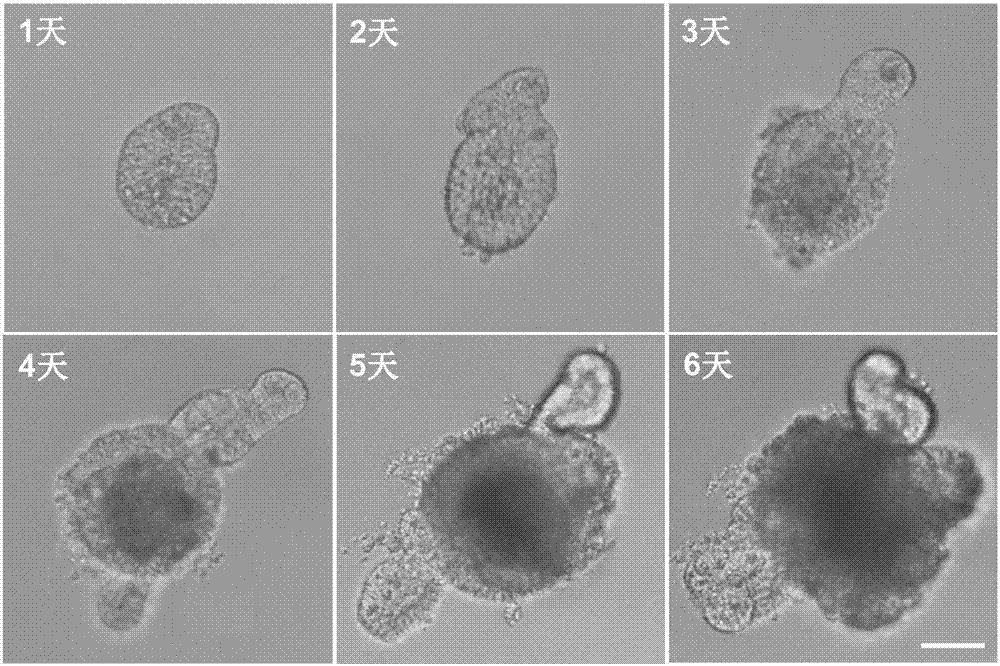
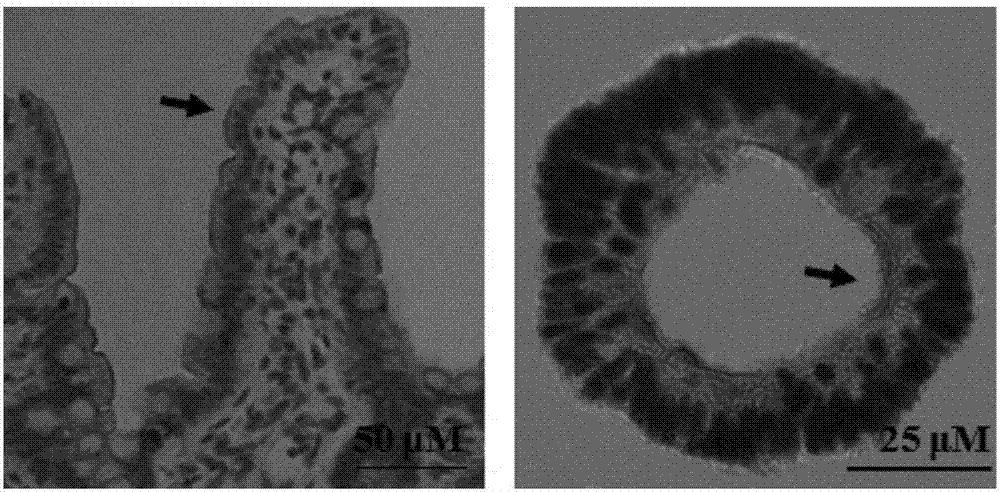
[0085] Using Olympus DP 71 inverted microscope to observe and photograph the process of 3D organoid formation on days 1, 2, 3, 4, 5 and 6 of culture, the results are as follows figure 1 showed that isolated crypts gradually differentiated into 3D organoids with increasing culture time, and stem cells in the crypts began to differentiate and "bud out" to form new crypt structures.
Embodiment 3
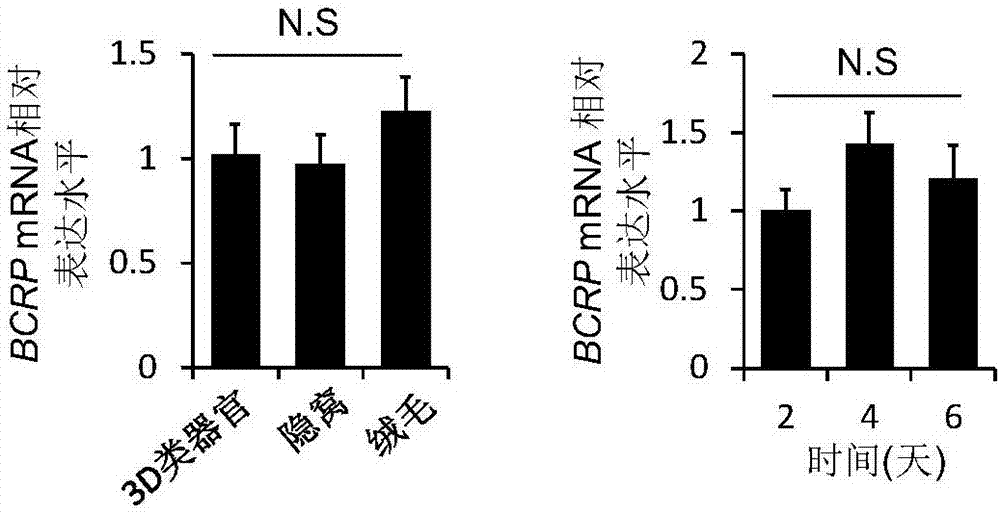
[0086] Example 3 Detection of BCRP mRNA level
[0087] (1) Isolation of small intestinal villi: According to the method for isolating crypts in Example 1, after step 5, small intestinal villi in a 70 μm cell mesh were collected.
[0088] (2) Isolation of small intestinal crypts: the mouse small intestinal crypts were obtained according to the separation method in Example 1.
[0089] (3) Acquisition of 3D organoids: small intestinal crypts were isolated and cultured for 4 days to form 3D organoids, the culture medium was discarded, washed twice with pre-cooled PBS, and then Matrigel was broken with a 1mL pipette tip, and it was washed with PBS. Transfer to a 1.5mL centrifuge tube, centrifuge at 200g for 5 minutes at 4°C, discard the supernatant, and precipitate into 3D organoids.
[0090] (4) mRNA extraction and reverse transcription: Trizol was used to extract total RNA from small intestinal villi, crypts and cultured 3D organoids, and cDNA was synthesized using a reverse tra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com