Anode material for lithium ion battery and preparation method thereof
A technology for lithium-ion batteries and cathode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problem of no significant effect on material structure stability, achieve improved reversible specific capacity, simple operation, and improved cycle stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
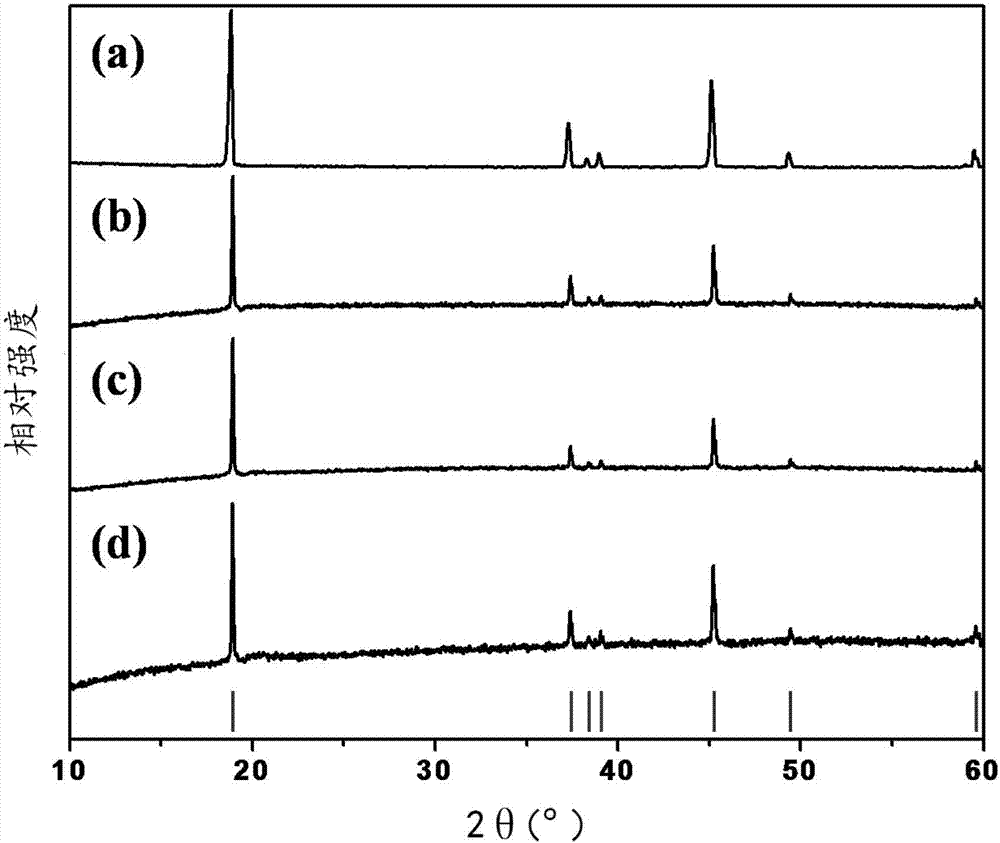
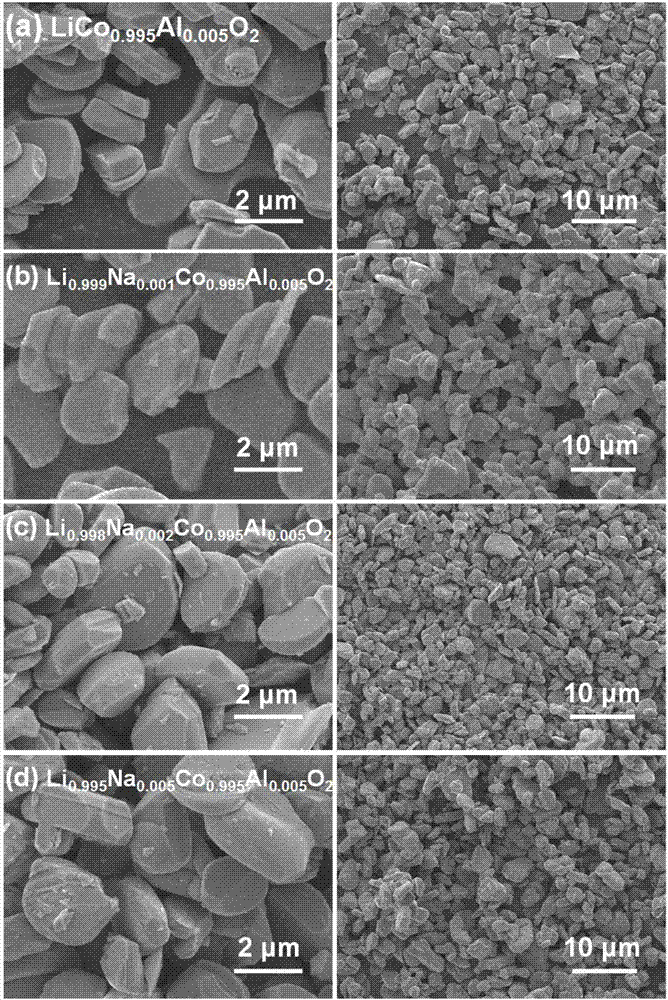
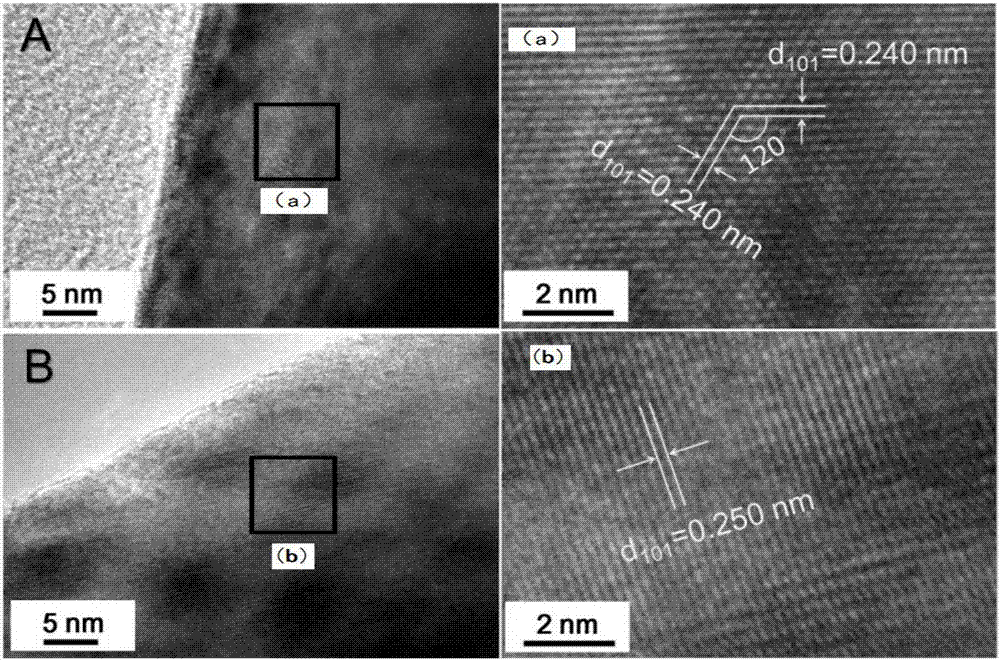
[0040] Weigh 22.1183g of 0.076mol of cobalt nitrate hexahydrate, 1.5005g of 0.004mol of aluminum nitrate nonahydrate, and 3g of polyacrylamide (PAM), and fully mix the above-mentioned weighed matter with 250mL of deionized water in a 1L beaker at one time. Sonicate until it is completely dissolved, which is solution A; weigh 0.082mol of sodium oxalate 10.988g, 0.001mol of oxalic acid 0.1261g, mix thoroughly with 250mL of deionized water in a 500mL beaker at one time, and ultrasonicate until completely dissolved, which is Solution B; place solution A in an oil bath at 80°C, adjust the speed to 800r / min, add solution B within 10 minutes, and continue stirring at a constant temperature for 2 hours; after the reaction, air cool to room temperature, filter and wash with water for 3 After washing once with alcohol, dry it in a forced air drying oven at 120°C for 4 hours to obtain the oxalate precursor. Weigh 20g of precursor and 4.1251g of lithium carbonate, mix them in a high-speed...
Embodiment 2
[0042] Weigh 22.1183g of 0.076mol of cobalt nitrate hexahydrate, 1.5005g of 0.004mol of aluminum nitrate nonahydrate, and 3g of polyacrylamide (PAM), and fully mix the above-mentioned weighed matter with 250mL of deionized water in a 1L beaker at one time. Sonicate until it is completely dissolved, which is solution A; weigh 0.082mol of sodium oxalate 10.988g, 0.001mol of oxalic acid 0.1261g, mix thoroughly with 250mL of deionized water in a 500mL beaker at one time, and ultrasonicate until completely dissolved, which is Solution B; place solution A in an oil bath at 80°C, adjust the speed to 800r / min, add solution B within 10 minutes, and continue stirring at a constant temperature for 2 hours; after the reaction, air cool to room temperature, filter and wash with water for 3 After washing once with alcohol, dry it in a forced air drying oven at 120°C for 4 hours to obtain the oxalate precursor. Weigh 15g of precursor, 3.0908g of lithium carbonate, 0.0044g of sodium carbonate...
Embodiment 3
[0044] Weigh 22.1183g of 0.076mol of cobalt nitrate hexahydrate, 1.5005g of 0.004mol of aluminum nitrate nonahydrate, and 3g of polyacrylamide (PAM), and fully mix the above-mentioned weighed matter with 250mL of deionized water in a 1L beaker at one time. Sonicate until it is completely dissolved, which is solution A; weigh 0.082mol of sodium oxalate 10.988g, 0.001mol of oxalic acid 0.1261g, mix thoroughly with 250mL of deionized water in a 500mL beaker at one time, and ultrasonicate until completely dissolved, which is Solution B; place solution A in an oil bath at 80°C, adjust the speed to 800r / min, add solution B within 10 minutes, and continue stirring at a constant temperature for 2 hours; after the reaction, air cool to room temperature, filter and wash with water for 3 After washing once with alcohol, dry it in a forced air drying oven at 120°C for 4 hours to obtain the oxalate precursor. Weigh 15g of precursor, 3.087g of lithium carbonate, 0.0088g of sodium carbonate,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com