Sirolimus nano-drug composition and preparation method thereof
A nano-drug, sirolimus technology, which is applied in the directions of drug combinations, pharmaceutical formulations, and non-active ingredients medical preparations, can solve the problems of unsuitability for large-scale industrialized continuous production, complex preparation processes, and high production costs, and achieves Easy to scale up and large-scale production, simple process, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
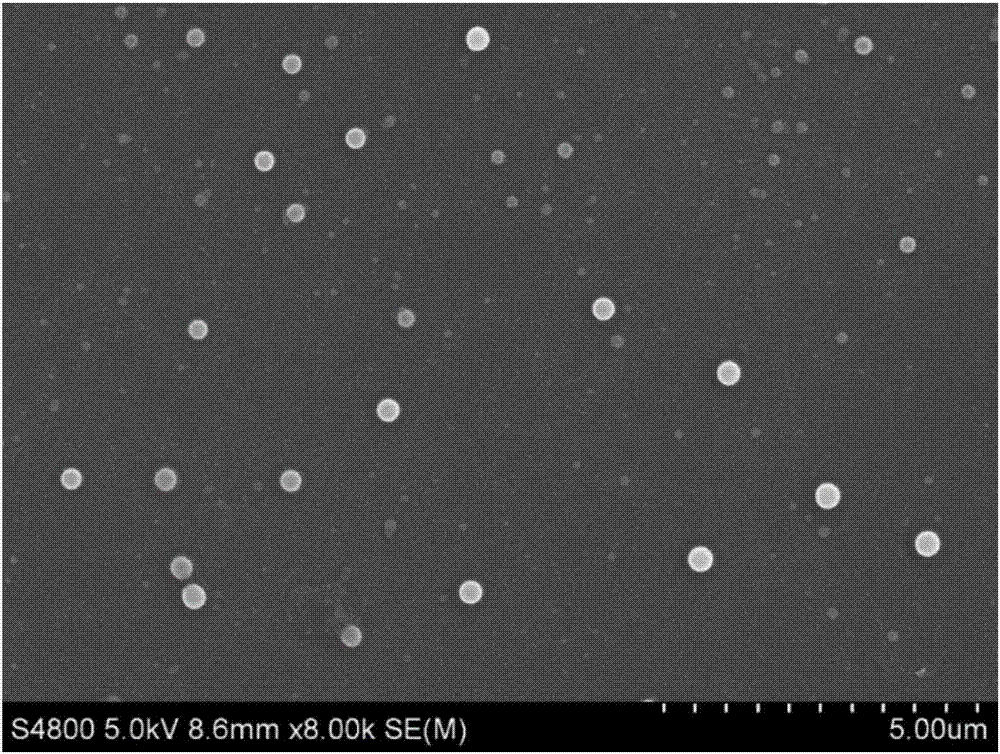
Image
Examples
Embodiment 1
[0049] A preparation method of sirolimus nano drug composition, comprising the steps of:
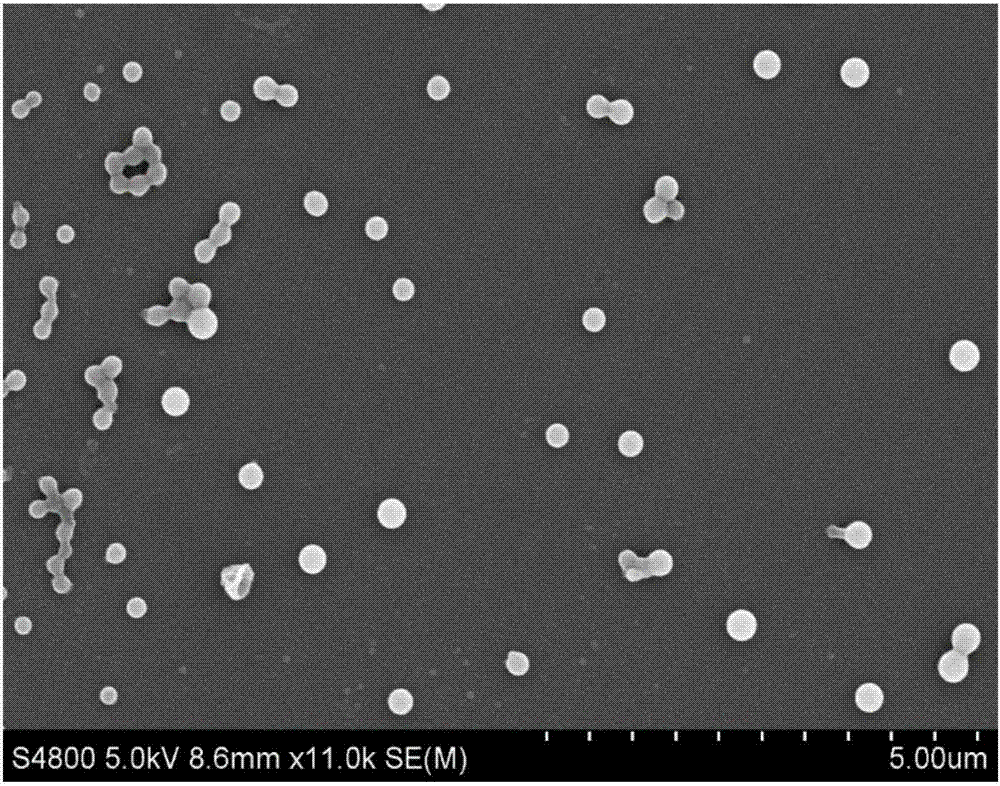
[0050] S01, the sirolimus bulk drug (scanning electron microscope picture as shown in figure 1 shown) with ethanol to prepare 10 mL of sirolimus ethanol solution with a concentration of 10 mg / mL; make polyvinylpyrrolidone and deionized water into 200 mL of 0.5 mg / mL adjuvant aqueous solution, and simultaneously add 5 mg of dodecyl dodecyl to the adjuvant aqueous solution sodium sulfate;
[0051] S02. Turn on the external circulation supergravity rotating packed bed, adjust the speed to 2272rpm; turn on the feed pump, and simultaneously transport sirolimus ethanol solution and auxiliary material aqueous solution into the supergravity rotating packed bed for recrystallization reaction, and control the sirolimus The feeding rate of the ethanol solution is 2mL / min, the feeding rate of the aqueous solution of the drug excipient is 40mL / min, and the temperature of the control system is 20°C t...
Embodiment 2
[0060] A preparation method of sirolimus nano drug composition, comprising the steps of:
[0061] S01, the sirolimus bulk drug and methanol are prepared into 10 mL of sirolimus ethanol solution with a concentration of 10 mg / mL; polyvinylpyrrolidone and deionized water are prepared into 200 mL of 0.5 mg / mL adjuvant aqueous solution;
[0062] S02. Turn on the external circulation supergravity rotating packed bed, adjust the speed to 2840rpm; turn on the feed pump, and simultaneously transport sirolimus ethanol solution and auxiliary material aqueous solution into the supergravity rotating packed bed for recrystallization reaction, and control the sirolimus The feed rate of ethanol solution is 5mL / min, the feed rate of pharmaceutical excipient aqueous solution is 50mL / min, and the temperature of the control system is 20°C to obtain the nano-suspension;
[0063] S03. Add mannitol and polyvinylpyrrolidone to the obtained nano-suspension, and then spray-dry to obtain a sirolimus nan...
Embodiment 3
[0066] A preparation method of sirolimus nano drug composition, comprising the steps of:
[0067] S01. Sirolimus bulk drug and dimethyl sulfoxide are made into 10 mL of sirolimus dimethyl sulfoxide solution with a concentration of 50 mg / mL; polyvinylpyrrolidone and deionized water are made into 8 mg / mL auxiliary materials 200mL aqueous solution;
[0068] S02. Turn on the external circulation supergravity rotating packed bed, adjust the rotating speed to 2250rpm; turn on the feed pump, and simultaneously transport sirolimus ethanol solution and auxiliary material aqueous solution into the supergravity rotating packed bed for recrystallization reaction, and control the sirolimus The feed rate of ethanol solution is 10mL / min, the feed rate of pharmaceutical excipient aqueous solution is 200mL / min, and the temperature of the control system is 20°C to obtain the nano-suspension;
[0069] S03. Add mannitol and polyvinylpyrrolidone to the obtained nano-suspension, and then spray-dry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com