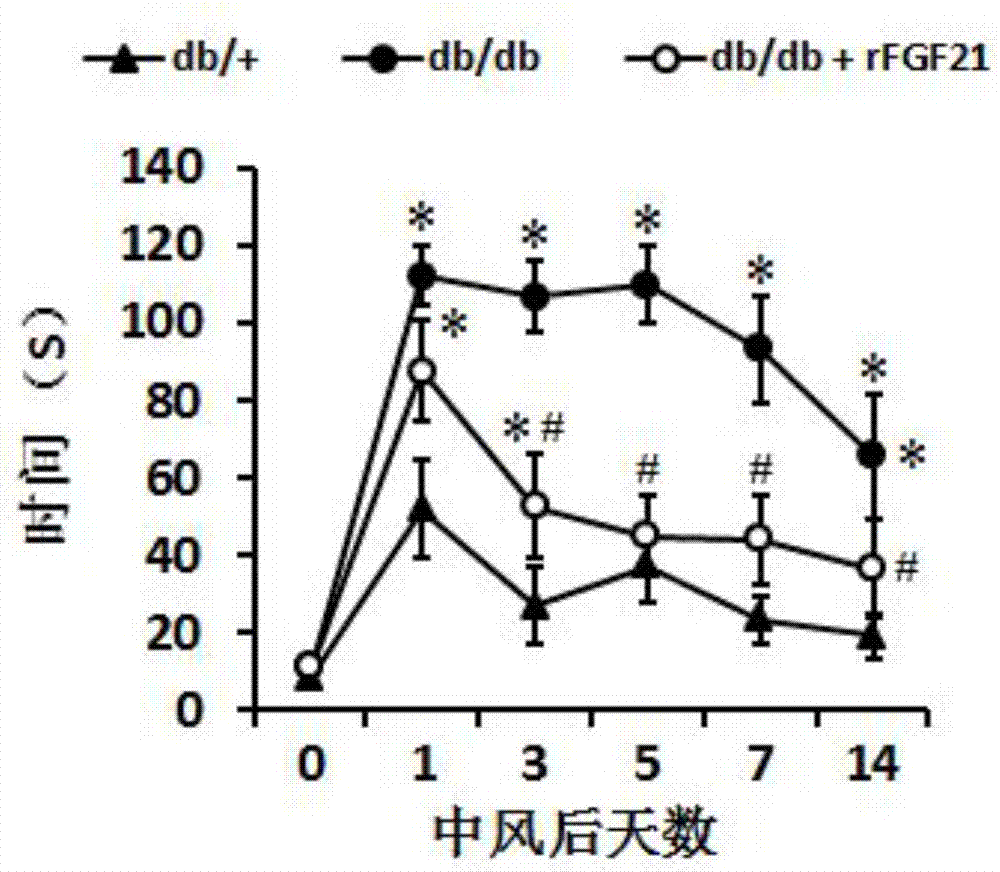
Application of human FGF21 (fibroblast growth factor 21) in preparing medicines for treating stroke
A growth factor and fibroblast technology, applied in the field of medicine, can solve the problems of little knowledge, limit the ability of brain nerve repair and reconstruction, and aggravate functional deficits, and achieve the effect of promoting nerve regeneration, promoting reconstruction, and reducing neuroinflammation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1 Obtaining exogenous recombinant human fibroblast growth factor 21
Embodiment 11
[0090] The construction of embodiment 1.1 high expression bacterial strain
[0091] Firstly, the corresponding DNA sequence was optimally designed according to the protein sequence of human FGF21 (NP_061986.1) on GenBank, as shown in SEQ ID NO.1. Subsequently, the corresponding DNA fragment was artificially synthesized, which had an NdeI restriction site at the 5' end and a BamHI restriction site at the 3' end. The resulting DNA of FGF21 was double digested with NdeI and BamHI, and the expression empty vector pET3c was treated with the same enzymes. The two digested fragments were ligated with T4 DNA ligase, transformed into competent Escherichia coli DH5α, and positive clones were screened for resistance to Ampicillin, and DNA sequence analysis was performed to ensure that the obtained cDNA sequence of FGF21 was correct. The resulting recombinant plasmid was named pET3c-FGF21. The recombinant plasmid pET3c-FGF21 was transformed into Escherichia coli BL21(DE3), and transform...
Embodiment 13
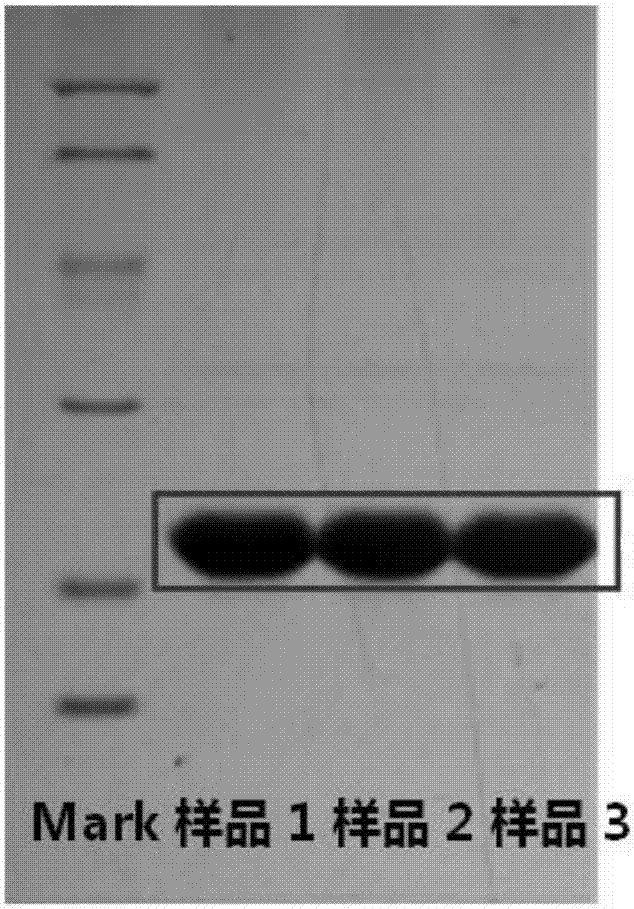
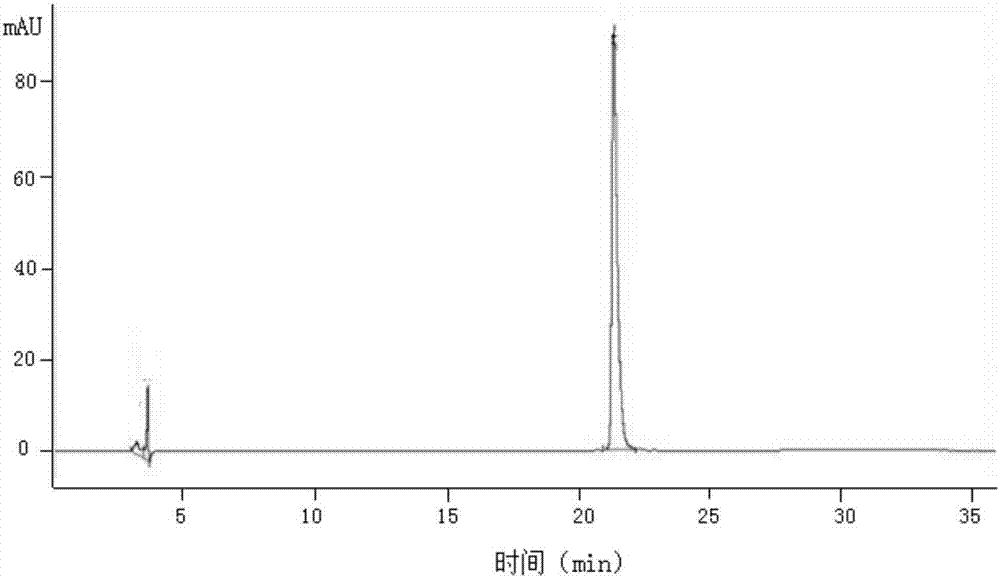
[0096] Example 1.3 Purification of FGF21
[0097] 1) Preparation of inclusion bodies
[0098] Suspend the wet cells in 10 times volume (w / v) buffer containing 25mmol / L Tris (pH 8.0), 150mmol / L NaCl, 10mmol / L EDTA-2Na; according to the required FGF21 wet cells, Weigh lysozyme and DNase at a ratio of 1:1000 (w / w) and 1:10000 (w / w), dissolve them in the lysate and add them to the bacterial suspension, stir and lyse at room temperature overnight, and microscopically check for cracks. Bacterial solution until there are no complete bacterial cells in the field of view, then use a high-speed refrigerated centrifuge (9000r / min, 4°C) to separate and collect the precipitate.
[0099] Suspend the precipitate from the previous step in 15 times the volume (w / v) of the wet cells containing 25mmol / L Tris (pH8.0), 150mmol / L NaCl, 10mmol / L EDTA-2Na, 0.2% deoxycholic acid In the sodium washing liquid I, fully stir to dissolve and mix well, then use a high-speed refrigerated centrifuge (9000r / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com