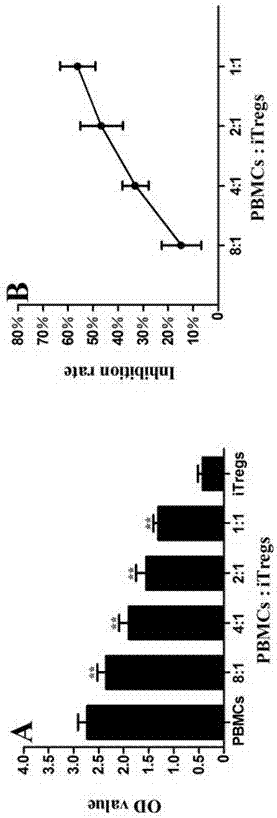
Application of MBL in preparing drug for preventing or treating disease induced by effector T cell
A technology for treating effects and diseases, applied in metabolic diseases, skin diseases, bone diseases, etc., to achieve the effect of strong regeneration ability and rich sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Experimental Materials
[0032] 1. Source of cells used in the experiment
[0033] Newborn umbilical cord blood was collected from the third affiliated hospital of Xinxiang Medical College and the full-term healthy newborns of the Department of Obstetrics and Gynecology of Xinxiang First People's Hospital. The blood was collected from the placenta umbilical vein immediately after delivery and put into a blood collection bag containing 28 mL of preservation solution. All blood samples were delivered to the laboratory within 4 hours after sampling.
[0034] 2. Main reagents
[0035] The medium RPMI1640, Fetal bovine serum (FBS), and 10×PBS solution were all purchased from ThermoFisher Scientific.
[0036] Lymphocyte separation solution (Ficoll-Paque PLUS) is a sterile endotoxin test solution (<0.12EU / mL) of FicollTM PM400 and sodium diatrizoate with a density of 1.077 g / mL, purchased from GE Healthcare Life Sciences.
[0037] Anti-human CD3 monoclonal antibody (CD3mAb), anti-human ...
Embodiment 2
[0094] Experimental Materials
[0095] 1. Source of cells used in the experiment
[0096] Reference example 1
[0097] 2. Main reagents
[0098] Reference example 1
[0099] 3. Main instruments and equipment
[0100] Reference example 1
[0101] 4. Preparation of common reagents
[0102] Reference example 1
[0103] experimental method
[0104] 1. Preparation of cord blood mononuclear cells
[0105] Reference example 1
[0106] 2. Coated with anti-CD3mAb
[0107] Reference example 1
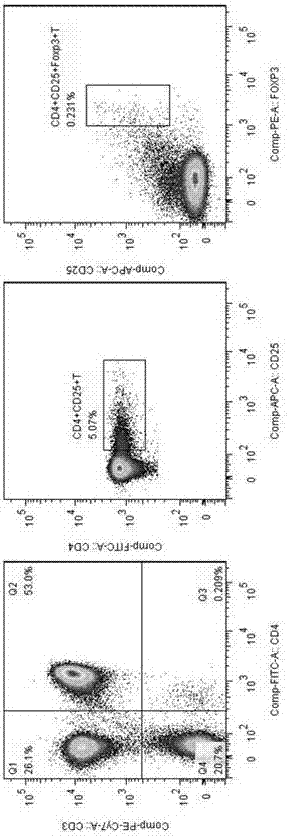
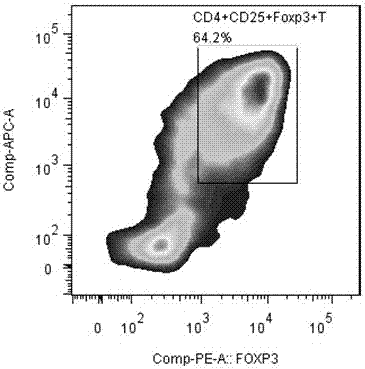
[0108] 3. Magnetic sorting of CD4+CD25-T cells and CD4+CD25+T cells
[0109] Sample preparation
[0110] Density gradient centrifugation was used to separate peripheral mononuclear cells. In order to remove platelets, the cells were resuspended in a buffer and centrifuged at 200×g for 10-15 minutes. The supernatant was carefully aspirated and washed repeatedly.
[0111] Magnetic labeling of non-CD4+ T cells
[0112] The entire process should be completed quickly and the cells should be kept in a pre-cooled state to pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com