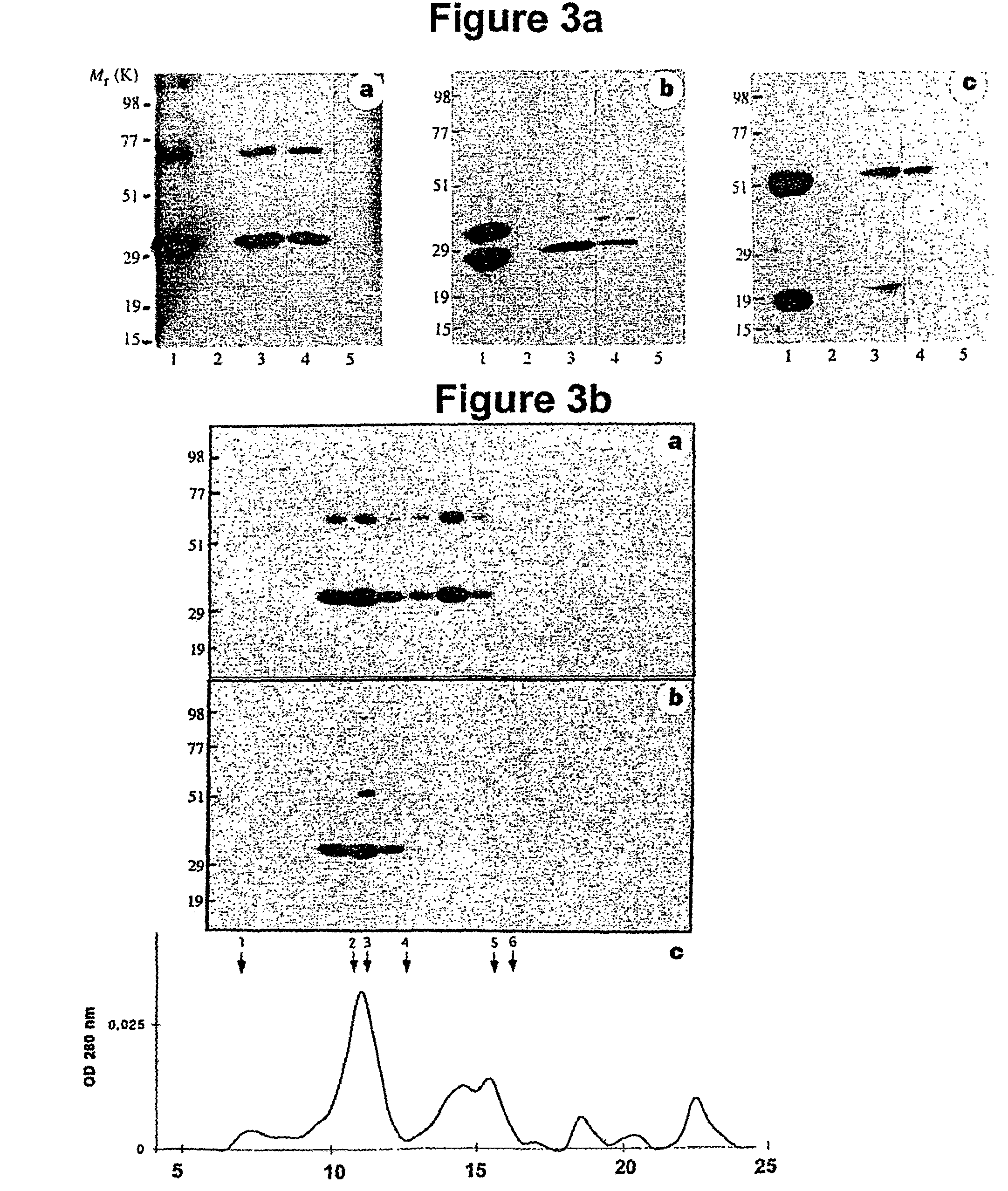
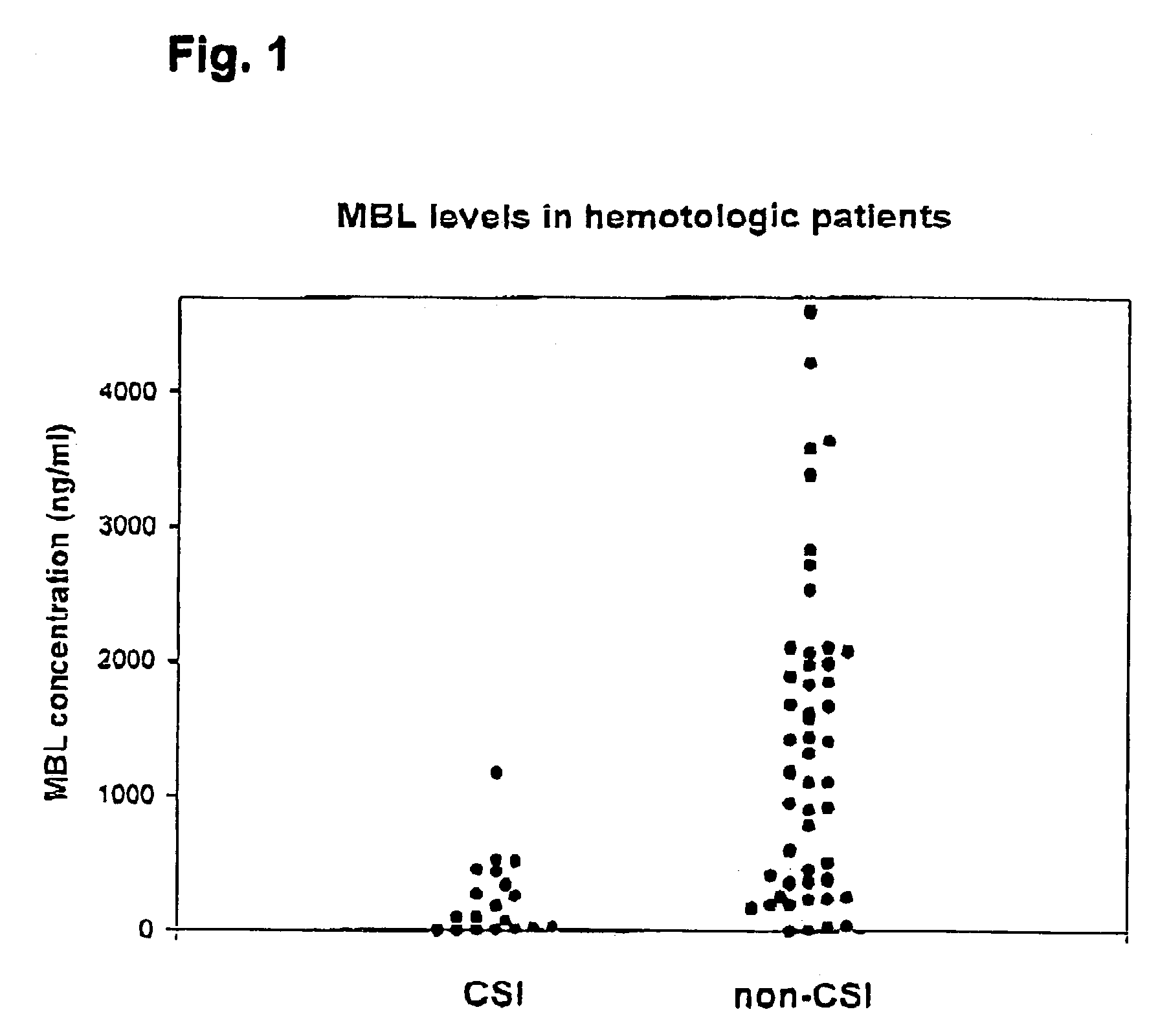
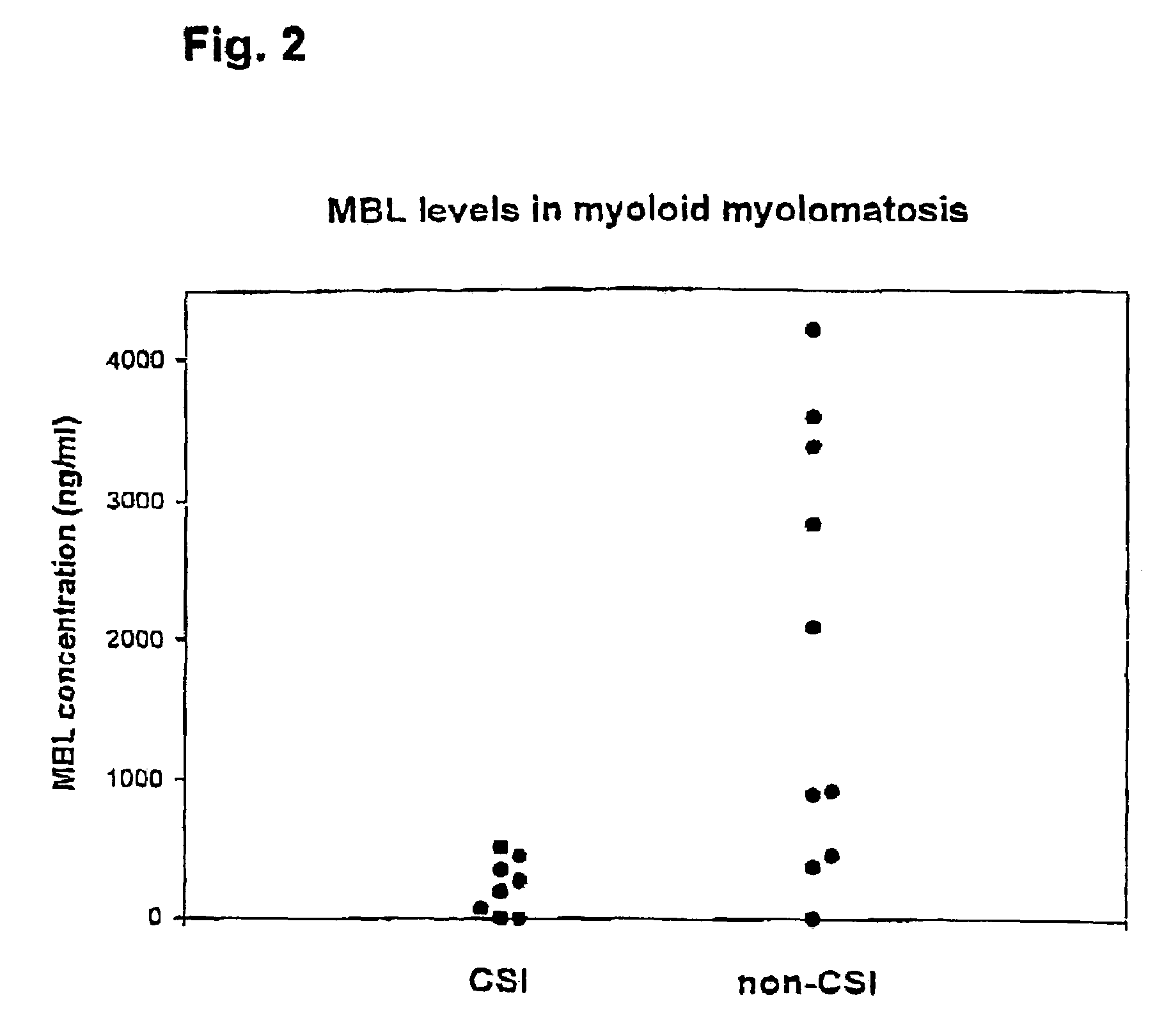
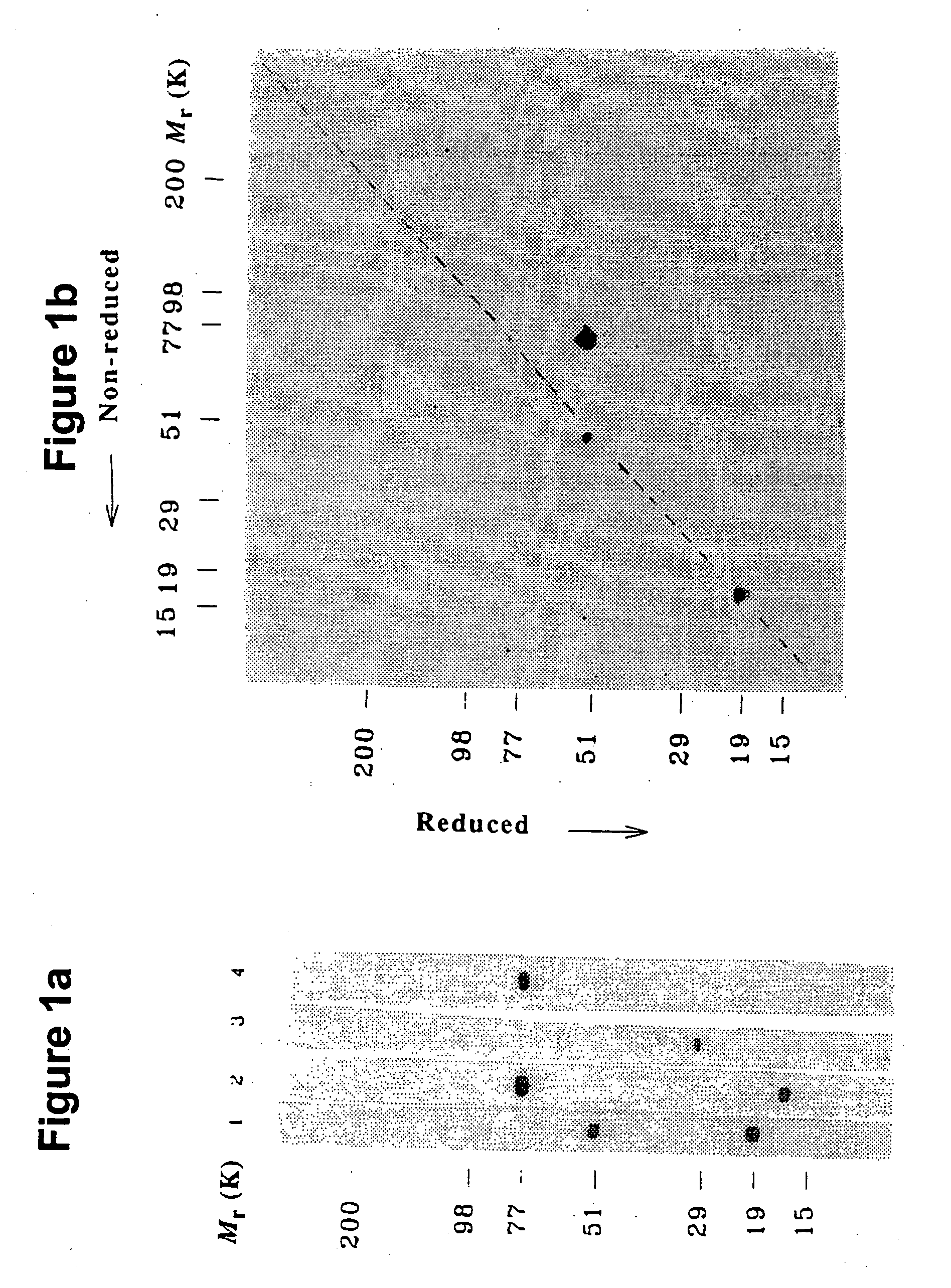
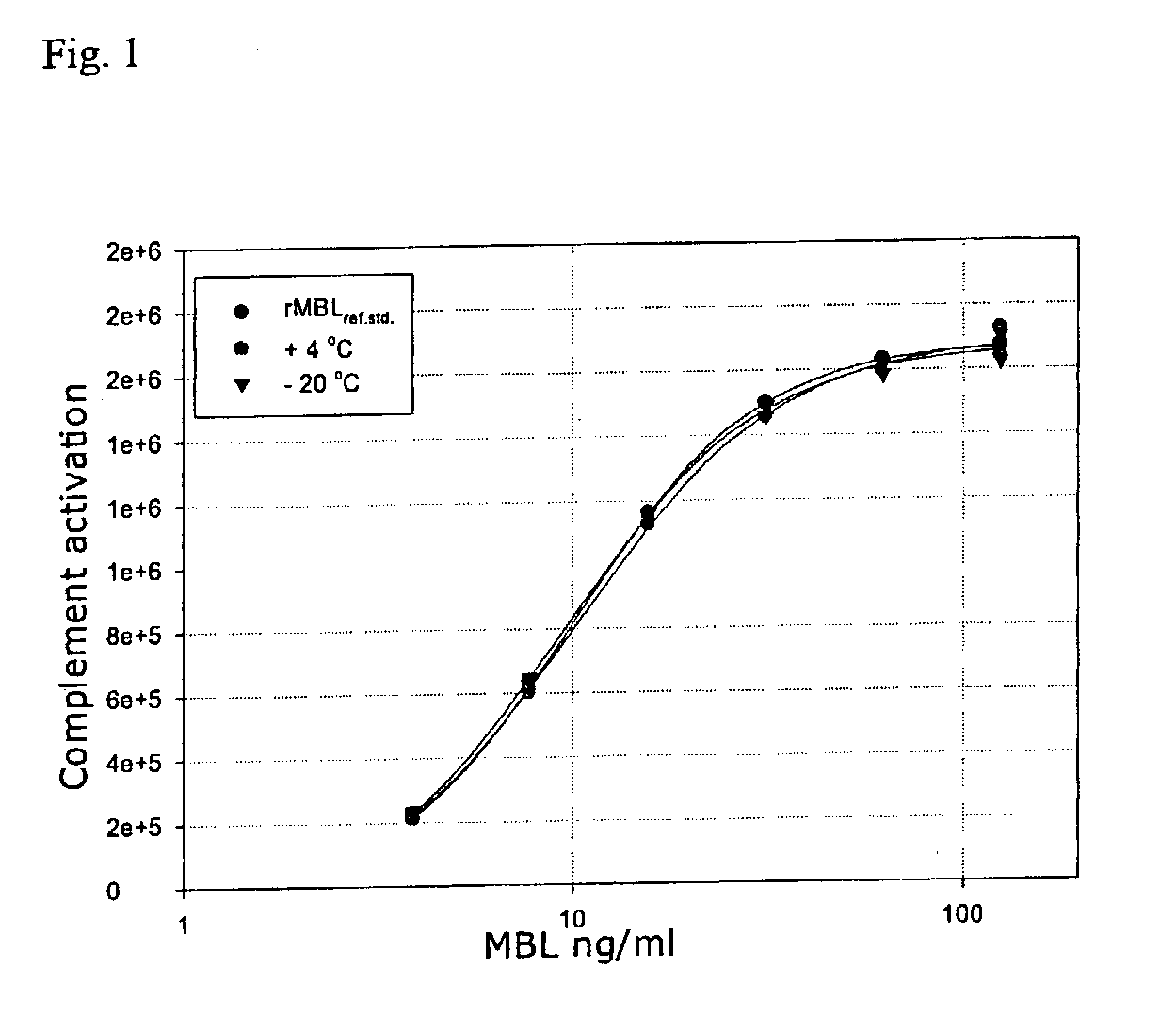
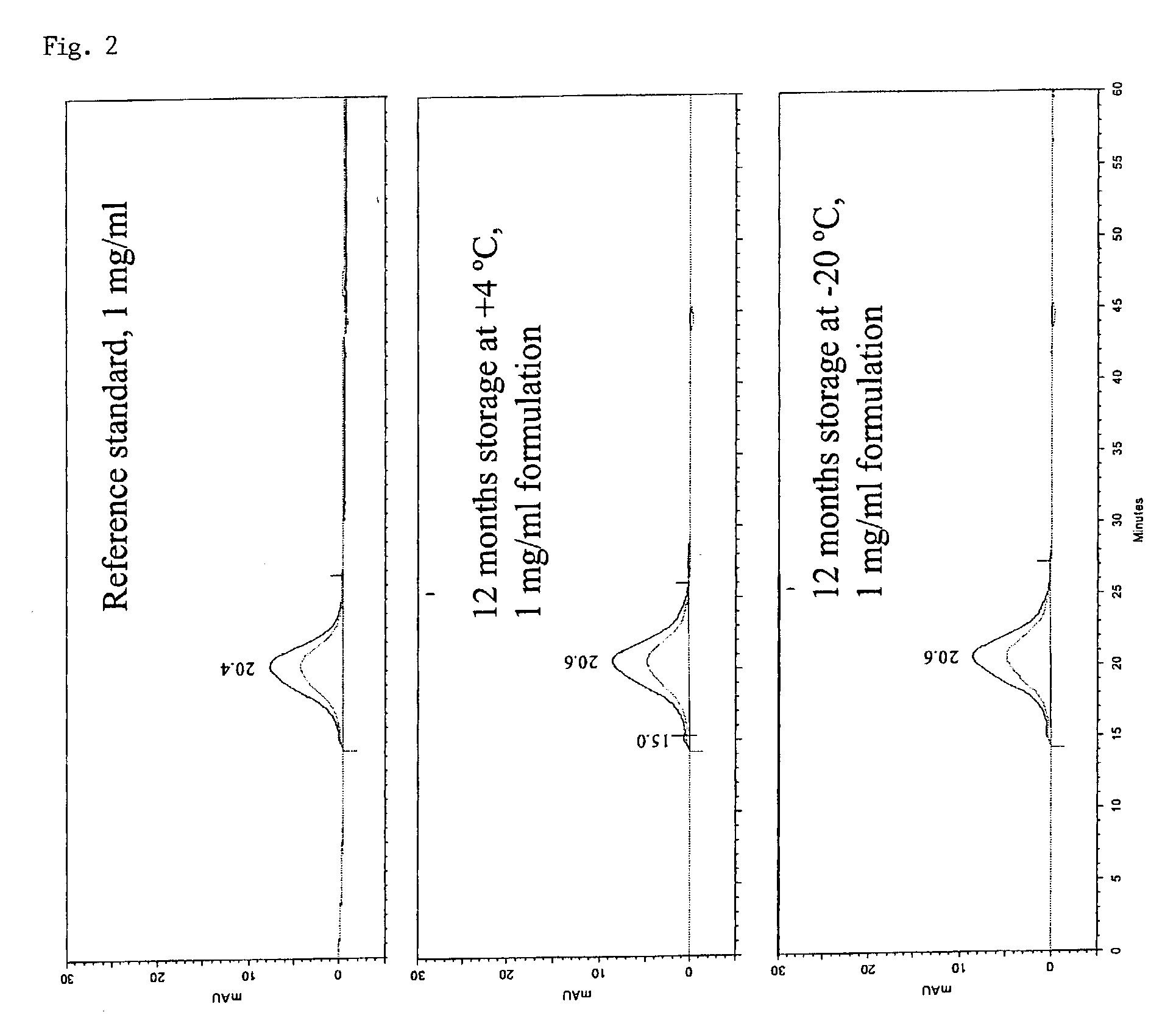
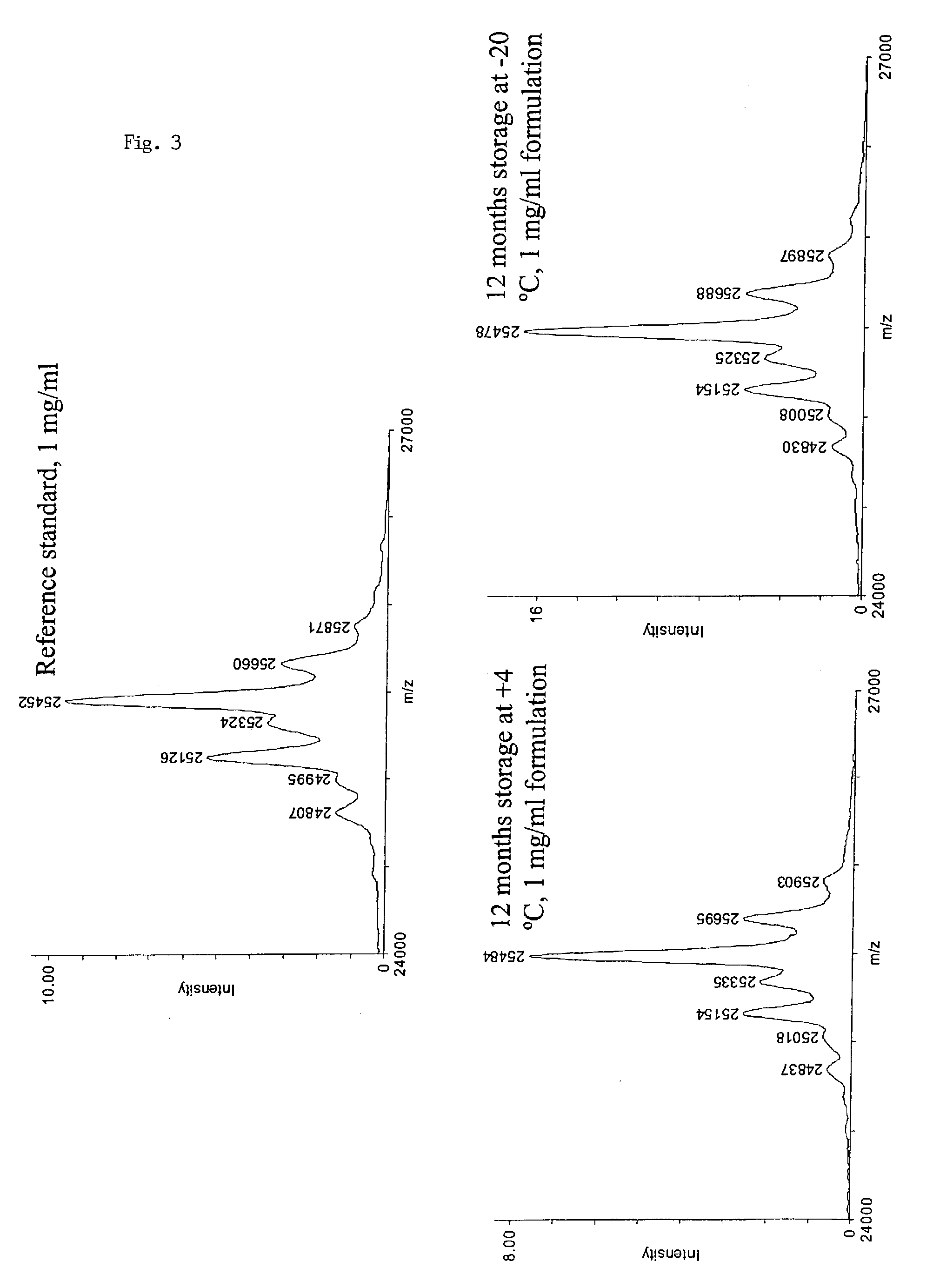
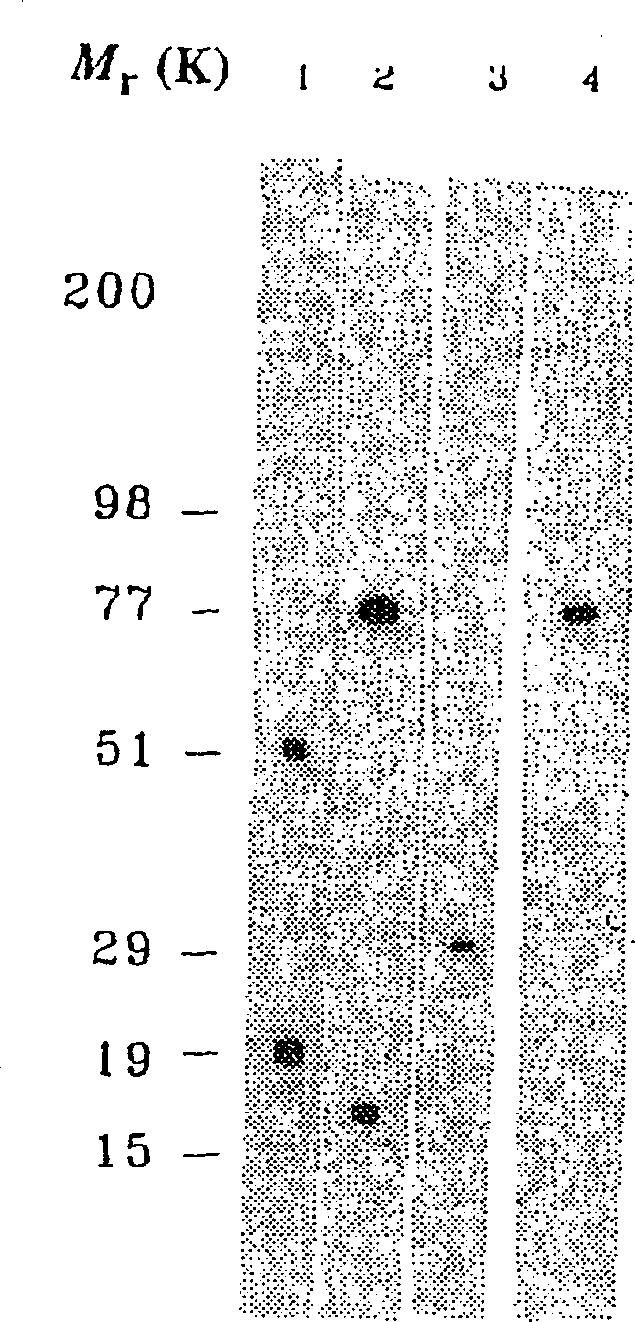
Patents
Literature
36 results about "Mannan-binding lectin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mannose-binding lectin (MBL), also called mannan-binding lectin or mannan-binding protein (MBP), is a lectin that is instrumental in innate immunity as an opsonin and via the lectin pathway.
Pharmaceutical compositions comprising mannose binding lectin
InactiveUS7462596B2Increase concentrationAntibacterial agentsAntimycoticsTotal proteinProtein materials
The present invention relates to pharmaceutical compositions comprising MBL and / or MBL variants. In particular the invention relates to pharmaceutical compositions comprising at least 200 μg / ml protein containing material, wherein mannan binding lectin (MBL) and / or MBL variants constitutes at least 35% (w / w) of the total protein; or to compositions comprising at least 400 μg / ml mannan binding lectin (MBL) and / or MBL variants. In addition the invention relates to pharmaceutical compositions comprising MBL and / or MBL variants and divalent cations. The invention also describes methods of preparing said compositions.The pharmaceutical compositions according to the invention may for example be used in methods of treatment of a number of different clinical conditions including infections. Uses of the compositions for preparation of medicaments for treatment of a clinical condition are also described.
Owner:NATLMMUNE AS (DK) +1
Masp-2, a complement-fixing enzyme, and uses for it
InactiveUS7112414B2Inhibition of activationOrganic active ingredientsHydrolasesEnzymeMannan-binding lectin
The present invention relates to substantially pure mannan-binding lectin associated serine protease-2 (MASP-2) polypeptides and fragments thereof as well as nucleic acids encoding such polpeptides. Furthermore, the present invention relates to uses of a substantially pure polypeptide comprising amino acid sequences derived from mannan-binding lectin associated serine protease-2 (MASP2) or a functional homologue thereof for the production of a pharmaceutical composition as well as pharmaceutical compositions comprising MASP-2 and / or MASP-2 fragments. In addition the present invention relates to inhibitors of MASP-2 and pharmaceutical compositions comparing such inhibitors. Methods for detecting MASP-2 nucleic acid expression are included in the invention.
Owner:HELION BIOTECH
Indications of mannan-binding lectin (MBL) in the treatment of immunocompromised individuals
InactiveUS7202207B2Reduce riskIncreased risk of infectionAntibacterial agentsOrganic active ingredientsOligomerImmunologic function
The present invention relates to the use of a composition comprising at least one mannan-binding lectin (MBL) subunit, or at least one mannan-binding lectin (MBL) oligomer comprising the at least one mannan-binding lectin (MBL) subunit, in the manufacture of a medicament for prophylaxis and / or treatment of infection. In particular the invention relates to prophylaxis and / or treatment of infection in an individual having an immunocompromised condition; and / or an individual being at risk of acquiring an immunocompromised condition resulting from a medical treatment. The present invention is particular relevant for prophylaxis and / or treatment of infection in individuals suffering from neutropenia, in particular as prophylaxis and / or treatment of infection in individuals receiving or going to receive chemotherapy or similar treatment. The individuals may be treated independent on their serum MBL level, and it has been shown that in particular individuals having a serum MBL level in the range of from 50 ng / ml serum to 500 ng / ml serum may benefit from the prophylaxis and / or treatment.
Owner:ENZON PHARM INC
Antibody pairs and kits for immunochemical determination of mannan-binding lectin
InactiveUS7211396B2Increased to exacerbationIncreased susceptibilityImmunoglobulins against animals/humansEnzymologyDiseaseAutoimmune disease
Various antibodies with different binding characteristics for MBL A or MBL B bound to mannan or MBL A or MBL B bound to an antibody against MBL are provided. Also provided are methods for selecting antibodies against MBL with different binding characteristics and methods and kits for using these antibodies to measure MBL capable of binding to mannan or oligomerized MBL and abnormal, poorly oligomerized MBL in a sample. The methods and kits are useful in diagnosing increased susceptibility to and exacerbation of infections and autoimmune diseases.
Owner:ANTIBODYSHOP
MASP-2, a complement-fixing enzyme, and uses for it
InactiveUS20050158297A1Increase and decrease expressionHigh expressionEgg immunoglobulinsBacteriaEnzymeMannan-binding lectin
Owner:UK RES & INNOVATION LTD +1
Method of predicting acute appendicitis
Embodiments of the invention provide method and devices for predicting the likelihood of acute appendicitis without invasive exploratory medical procedures. Several protein biomarkers: leucine-rich α-2-glycoprotein (LRG); S100-A8 (calgranulin); α-1-acid glycoprotein 1 (ORM); plasminogen (PLG); mannan-binding lectin serine protease 2 (MASP2); zinc-α-2-glycoprotein (AZGP1); Apolipoprotein D (ApoD); and α-1-antichymotrypsin (SERPINA3); are increased in the urine of patients with appendicitis. The method and devices comprise detecting the levels of these biomarkers and comparing with reference levels found in healthy individuals.
Owner:CHILDRENS MEDICAL CENT CORP
Pharmaceutical compositions comprising mannose binding lectin
InactiveUS20040006009A1Increase concentrationAntibacterial agentsBiocideTotal proteinMannose binding
The present invention relates to pharmaceutical compositions comprising MBL and / or MBL variants. In particular the invention relates to pharmaceutical compositions comprising at least 200 mug / ml protein containing material, wherein mannan binding lectin (MBL) and / or MBL variants constitutes at least 35% (w / w) of the total protein; or to compositions comprising at least 400 mug / ml mannan binding lectin (MBL) and / or MBL variants. In addition the invention relates to pharmaceutical compositions comprising MBL and / or MBL variants and divalent cations. The invention also describes methods of preparing said compositions. The pharmaceutical compositions according to the invention may for example be used in methods of treatment of a number of different clinical conditions including infections. Uses of the compositions for preparation of medicaments for treatment of a clinical condition are also described.
Owner:NATLMMUNE AS (DK) +1
Masp-2, a complement-fixing enzyme, and uses for it
The present invention relates to substantially pure mannan-binding lectin associated serine protease-2 (MASP-2) polypeptides and fragments thereof as well as nucleic acids encoding such polpeptides. Furthermore, the present invention relates to uses of a substantially pure polypeptide comprising amino acid sequences derived from mannan-binding lectin associated serine protease-2 (MASP2) or a functional homologue thereof for the production of a pharmaceutical composition as well as pharmaceutical compositions comprising MASP-2 and / or MASP-2 fragments. In addition the present invention relates to inhibitors of MASP-2 and pharmaceutical compositions comparing such inhibitors. Methods for detecting MASP-2 nucleic acid expression are included in the invention.
Owner:詹斯·C·詹斯尼厄斯 +1
Compositions, methods and kits for immunochemical determination of mannan-binding lectin (MBL)
InactiveCN1646916AIncreased susceptibilityImmunoglobulins against animals/humansEnzymologyDiseaseMannan binding
Owner:ANTIBODYSHOP
Mannan-binding lectin (MBL) in the treatment of immunocompromised conditions asssociated with cancer
The present invention pertains to the use of subunits and oligomers of mannan- binding lectin (MBL) in prophylactic and / or curative treatment of an immunocompromised individual such as subjects suffering from solid tumors or haematological cancers. Solid tumors include such as female cancers, male cancers, cancers of the respiratory system, cancers of the gastro intestinal system, the renal system and further subjects suffering from thyroid cancer and melanomas. Haematological cancers include leukaemia, lymphoma and myeloma. The immunocompromised condition of the individual may be due to a cancer disease as mentioned herein or the treatment of said cancer disease.
Owner:内蒂穆恩公司
Method for detecting mycoplasma pneumonia (MP) of sheep
InactiveCN102363804AVerify dependenciesScreen sensitive and efficientMicrobiological testing/measurementMicroorganism based processesSusceptible individualBisulfite sequencing
The invention which belongs to the technical field of biological detection relates to a method for detecting MP of sheep and provides the methylation state of CpG islands in an MBL (mannan-binding lectin) gene promoter region related with the MP of sheep and an application thereof. The invention also provides a method for detecting the MP of sheep and concretely relates to the methylation detection of the MBL promoter region and the artificial infection verification. The methylation state detection of the MBL gene promoter region of sheep is carried out through selecting a bisulfite sequencing process, the methylation state and the frequency of each CpG locus in a given region can be accurately detected, three substantially differential CpG methylation loci, CpG3, CpG4 and CpG5, related with the MBL level are found, a case that the MBL level after toxic elimination is lower than the MBL level before the toxic elimination is promoted, and the MBL gene promoter region methylation may be a more substantial characteristic of the MBL level reduction of serum. The method allows the MP infection of sheep and susceptible individuals to be sensitively and accurately detected, and provides complete information and scientific bases for the sheep MBL gene promoter region methylation and the MP infection research of sheep.
Owner:赵宗胜
Novel Cancer Indications of Mannan-Binding Lectin (Mbl) in the Treatment of Immunocompromised Individuals
InactiveUS20080214435A1Sufficient immune responseAntibacterial agentsAntimycoticsImmune compromisedOncology
The present invention pertains to the use of subunits and oligomers of mannan-binding lectin (MBL) in prophylactic and / or curative treatment of an immunocompromised individual such as subjects suffering from solid tumors or haematological cancers. Solid tumors include such as female cancers, male cancers, cancers of the respiratory system, cancers of the gastro intestinal system, the renal system and further subjects suffering from thyroid cancer and melanomas. Haematological cancers include leukaemia, lymphoma and myeloma. The immunocompromised condition of the individual may be due to a cancer disease as mentioned herein or the treatment of said cancer disease.
Owner:NAT IMMUNE
Recombinant human mannan-binding lectin
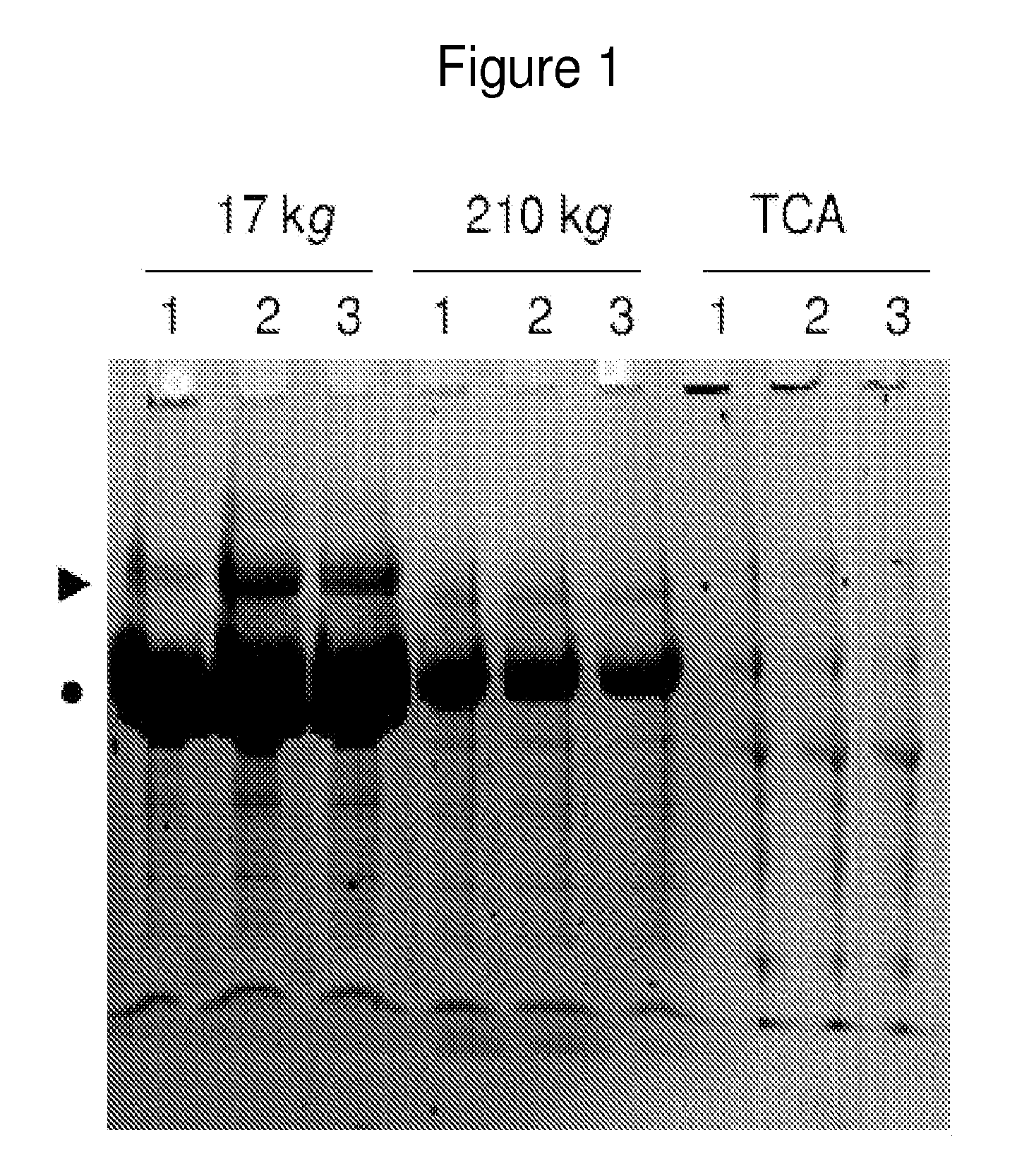
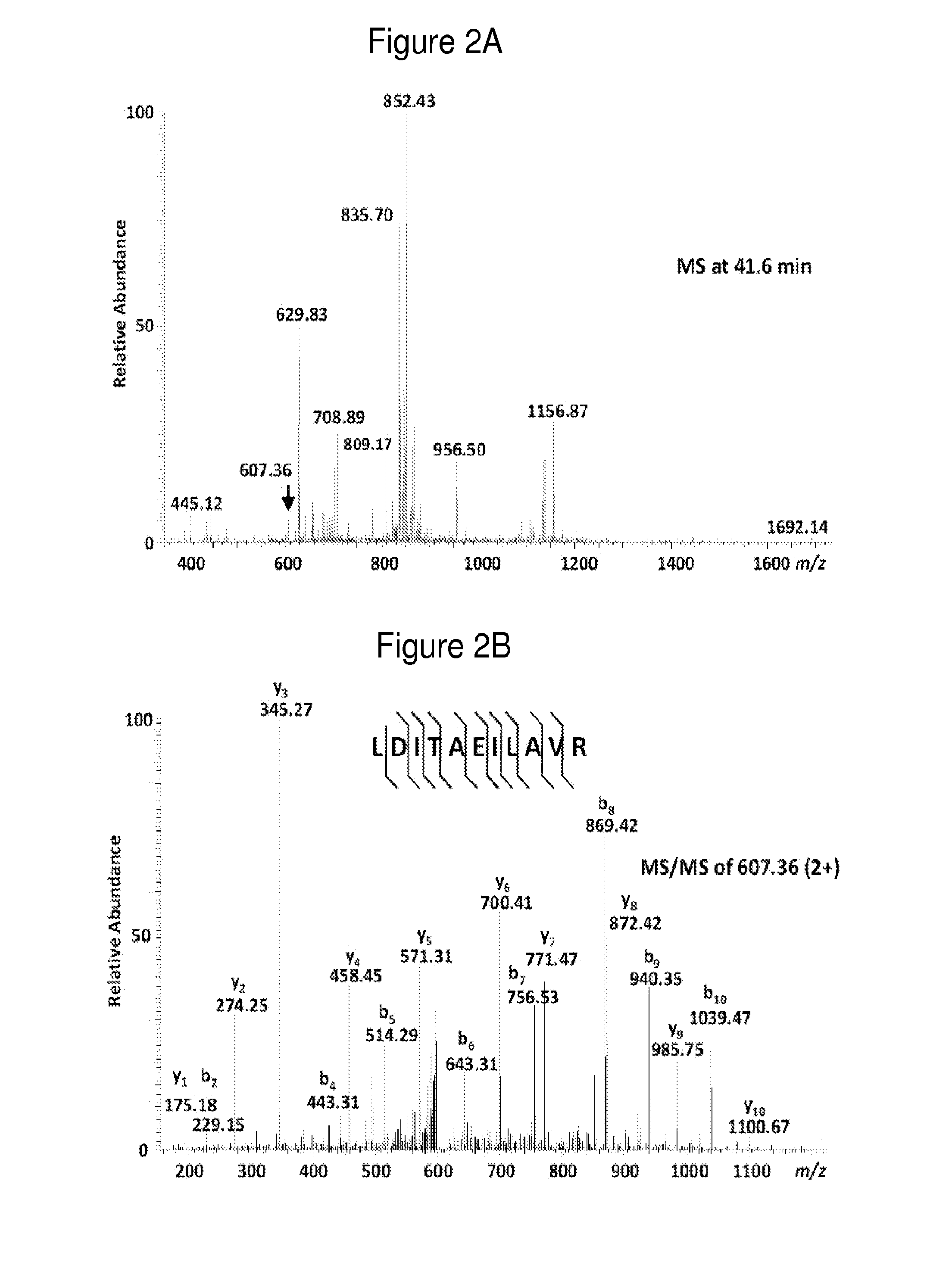
The invention relates to treatment on immune system diseases which includes the treatment on the defect in the immune system. The invention includes the preparation of a novel expressing construction body for coding the MBL of human, a novel preparation of recomposing the MBL of human with a high similarity to a natural MBL of human and also includes the application of the compound in combinations, drugs and treatment on the state correlative with an immune restraining state and / or the MBL defect state (including a latent state). An MBL defect state is correlative with the susceptibility increase of infection. More concretely, the invention relates to the treatment on the state (including the latent state, namely the state needs not to be treated currently). The treatment aims at human beings and animals the immune systems of which are similar to human beings.
Owner:斯蒂芬·蒂尔 +2
Methods for identifying inhibitors of mannan-binding lectin associated serine protease (MASP) proteins and uses thereof
ActiveUS20120225437A1Inhibitory activityOrganic active ingredientsCompound screeningScreening methodProtein activity
This disclosure is directed to methods and compositions to inhibit MASP protein activity using small molecule inhibitors. In one aspect, the disclosure is directed to methods for identifying inhibitors of MASP protein activity, including methods of screening capable of inhibiting MASP protein activity.
Owner:UNIVERSITY OF LEICESTER
Method for early diagnosing acute kidney injury caused by contrast medium by utilizing MBL (mannan-binding lectin)
InactiveCN101995428AMicrobiological testing/measurementMaterial analysis by electric/magnetic meansSerum creatineCreatinine rise
The invention relates to a method for early diagnosing an acute kidney injury caused by a contrast medium by utilizing urine MBL (mannan-binding lectin), which comprises the following steps of: comparing fluorescence difference electrophoresis charts of the urine of a patient 24 hours after an operation and the basis urine of the patient before the operation, and searching out a plurality of differentially expressed protein spots; identifying through a matrix-supported laser desorption ionization time of flight mass spectrometry technique to discover that the mannan-binding lectin (MBL) and serine protease related to the MBL are both increased in the differentially expressed protein spots, and the difference has a statistical significance for prompting that the acute kidney injury caused by the contrast medium is related to a pathway of MBL (mannan-binding lectin) complement activation, which is verified by a patient with the kidney injury caused by the contrast medium through an urine ELISA (enzyme-linked immuno sorbent assay) way. If applied to the clinic, the method of the invention can be used for detecting the urine MBL (mannan-binding lectin) 24 hours after an operation through the ELISA (enzyme-linked immuno sorbent assay) way and early diagnosing the kidney injury caused by the contrast medium, which is 24 hours earlier than the diagnosis of serum creatinine, and provides basis for early clinical diagnosis, early treatment and improvement of prognosis.
Owner:贾明宏
Methods and products for regulating lectin complement pathway associated complement activation
InactiveUS7273925B1Inhibiting LCP-associated complement activationAvoid depositionAnimal cellsImmunoglobulins against animals/humansEpitopeBinding peptide
The invention relates to methods and products for regulating lectin complement pathway associated complement activation. The methods include both in vitro and in vivo methods for inhibiting lectin complement pathway associated complement activation. The methods are accomplished by contacting a mammalian cell having surface exposed MBL ligand with an effective amount of a mannan binding lectin inhibitor to inhibit lectin complement pathway associated complement activation. The mannan binding lectin inhibitor may be administered to a subject to prevent cellular injury mediated by lectin complement pathway associated complement activation. The products of the invention include compositions of a mannan binding lectin inhibitor. The mannan binding lectin inhibitor is an isolated mannan binding lectin binding peptide that selectively binds to a human mannan binding lectin epitope and that inhibits lectin complement pathway associated complement activation. The products also include hybridoma cell lines and pharmaceutical compositions.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
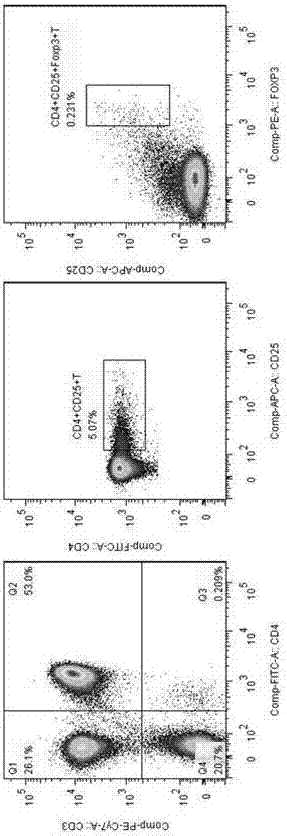
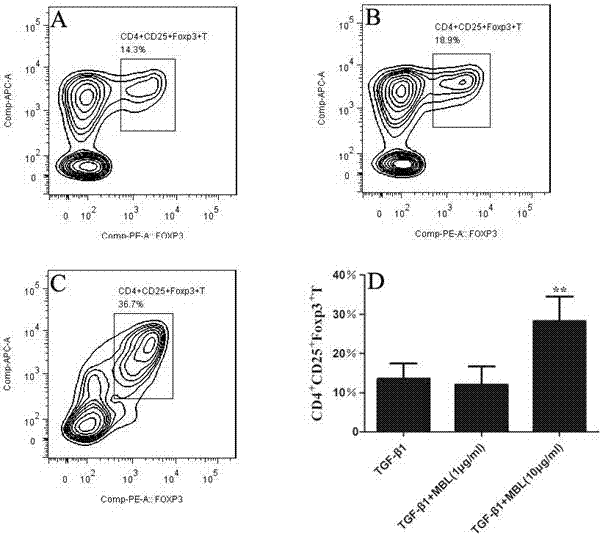
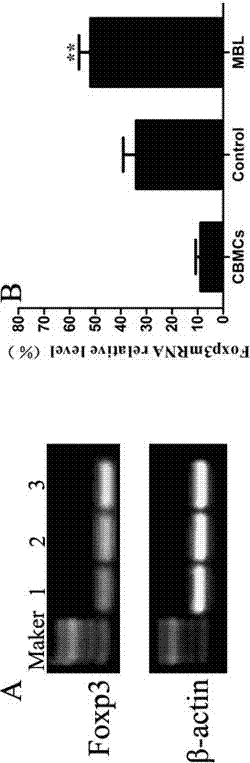
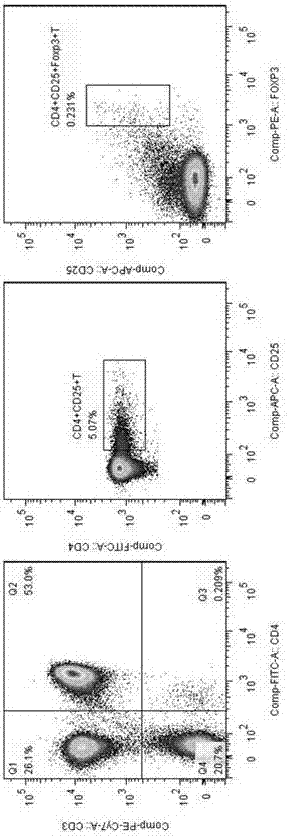
Application of MBL in preparing drug for preventing or treating disease taking Tregs as target spot
PendingCN107050427AIncrease the number ofPromote induced differentiationPeptide/protein ingredientsAntipyreticAutoimmune diseaseAllergy disorders
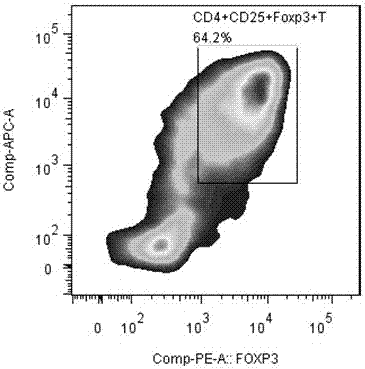
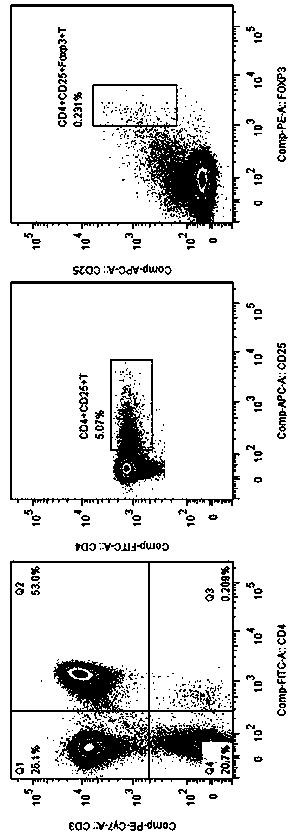
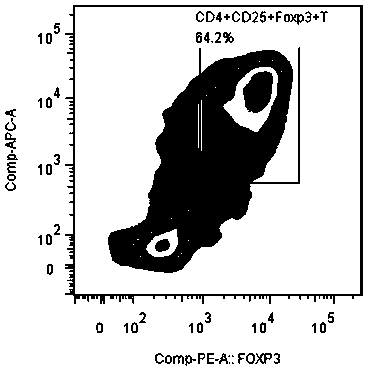
The invention discloses an application of MBL in preparing a drug for preventing or treating disease taking Tregs as a target spot and belongs to the technical field of a novel application of mannan binding lectin. The key point of the technical scheme is as follows: the invention relates to the application of MBL in preparing a drug for preventing or treating infectious diseases, autoimmune diseases or / and allergic diseases induced by taking Tregs as the target spot, wherein MBL realizes functions of the drug by accelerating induced differentiation of a CD4<+>CD25-T cell toward a Tregs cell. Through exploration, a novel factor of adjusting induced differentiation of the Tregs cell is obtained, that is, MBL directly enhances induced differentiation of the CD4<+>CD25-T cell toward the Tregs cell. The application disclosed by the invention highlights a novel function of MBL, and MBL not only plays an important anti-infection role in inherent immunity, but also can be a pertinent target spot for treating various autoimmune diseases and inflammatory diseases in intervention of induced differentiation of the Tregs cell.
Owner:XINXIANG MEDICAL UNIV +1
Application of MBL in preparing drug for preventing or treating disease induced by effector T cell
ActiveCN107050436AEnhanced inhibitory effectRich sourcesAntibacterial agentsPeptide/protein ingredientsAutoimmune diseaseAllergy disorders
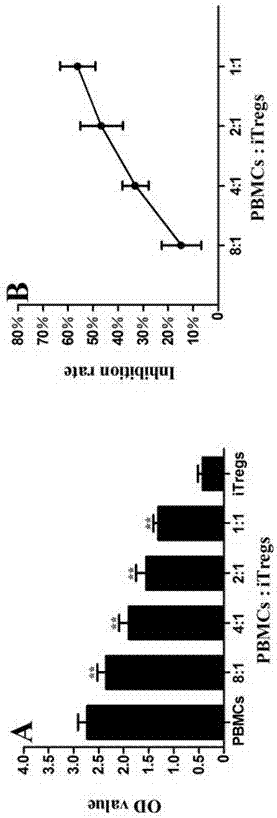
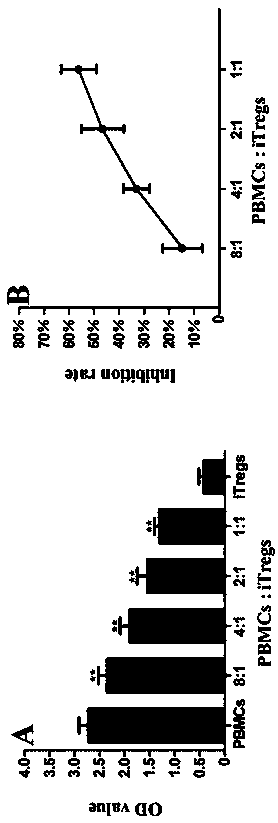
The invention discloses an application of MBL in preparing a drug for preventing or treating disease induced by an effector T cell and belongs to the technical field of a novel application of mannan binding lectin. The key point of the technical scheme is as follows: the invention relates to the application of MBL in preparing a drug for preventing or treating infectious diseases, autoimmune diseases or / and allergic diseases induced by the effector T cell, wherein MBL realizes functions of the drug by enhancing iTreg cell and inhibiting proliferation of the effector T cell. Through researches, a novel factor of adjusting the inhibition function of the Tregs cell is obtained, that is, MBL directly enhances the inhibition action of the Tregs cell on proliferation of the effector T cell. The application disclosed by the invention highlights a novel function of MBL, and MBL not only plays an important anti-infection role in inherent immunity, but also can be a pertinent target spot for treating various autoimmune diseases and inflammatory diseases in intervention of the function of the Tregs cell.
Owner:XINXIANG MEDICAL UNIV
Method of predicting acute appendicitis
Embodiments of the invention provide method and devices for predicting the likelihood of acute appendicitis without invasive exploratory medical procedures. Several protein biomarkers: leucine-rich α-2-glycoprotein (LRG); S100-A8 (calgranulin); α-1-acid glycoprotein 1 (ORM); plasminogen (PLG); mannan-binding lectin serine protease 2 (MASP2); zinc-α-2-glycoprotein (AZGP1); Apolipoprotein D (ApoD); and α-1-antichymotrypsin (SERPINA3); are increased in the urine of patients with appendicitis. The method and devices comprise detecting the levels of these biomarkers and comparing with reference levels found in healthy individuals.
Owner:CHILDRENS MEDICAL CENT CORP
Serum components that bind to threat agents
InactiveUS20090166200A1Sludge treatmentVolume/mass flow measurementConjugate vaccineProtective antigen
Low molecular weight serum components (less than 10,000 m.w.), in vaccinated animals and a human subject who has been exposed to a threat agent inadvertently, bound to purified O-polysaccharide (OPS, a polymer of formamido-mannose) and a candidate of a threat agent, such as Brucella suis 145 vaccine is disclosed. These components formed a loose reversible precipitin with OPS in a high-salt borate-buffered agarose gel and bound to the candidate vaccine as observed by capillary electrophoresis. By using modified capillary electrophoresis, the invention also discloses the presence of two larger serum components, one similar in size to that of serum albumin and one resembles that of mannan-binding lectin, that bound to the vaccine. An indirect method for identifying vaccination is the presence of antibodies against Brucella-OPS-antibodies. ELISA, capillary electrophoresis and animal challenge studies showed that as high as 30% of the control animals did not require vaccination. These animals could have been exposed to cross-reactive cross-protective antigens naturally.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Treating Infection
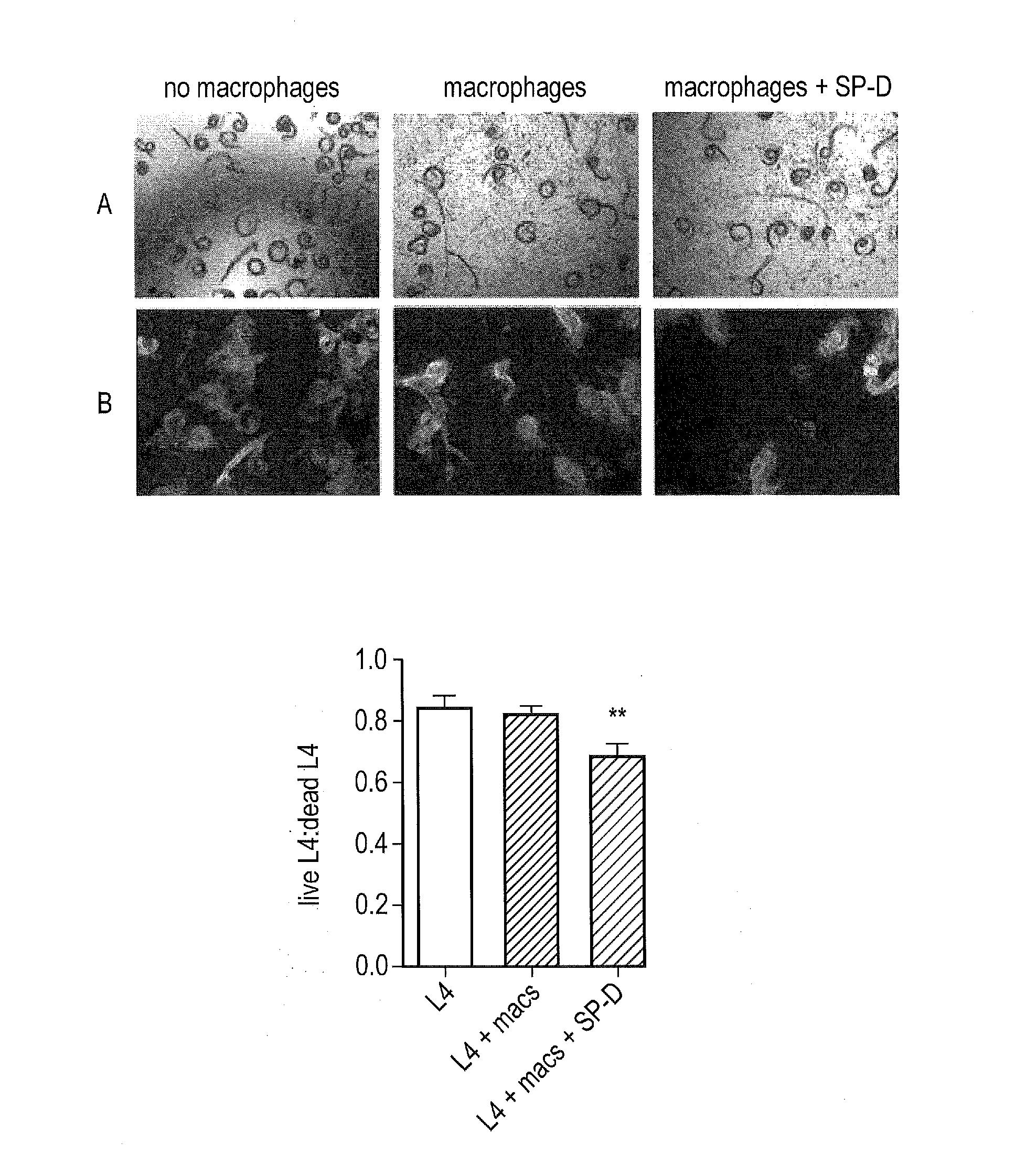
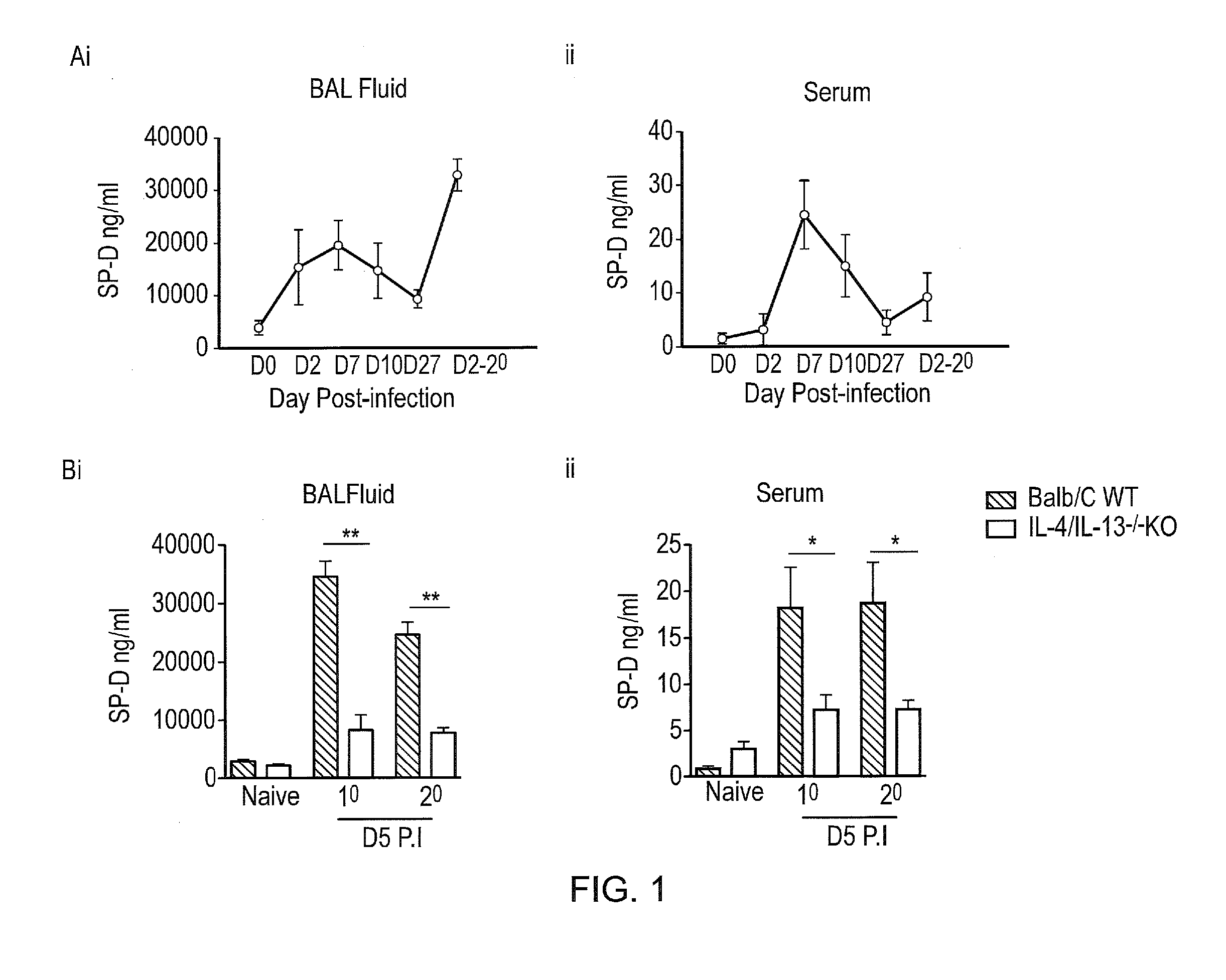
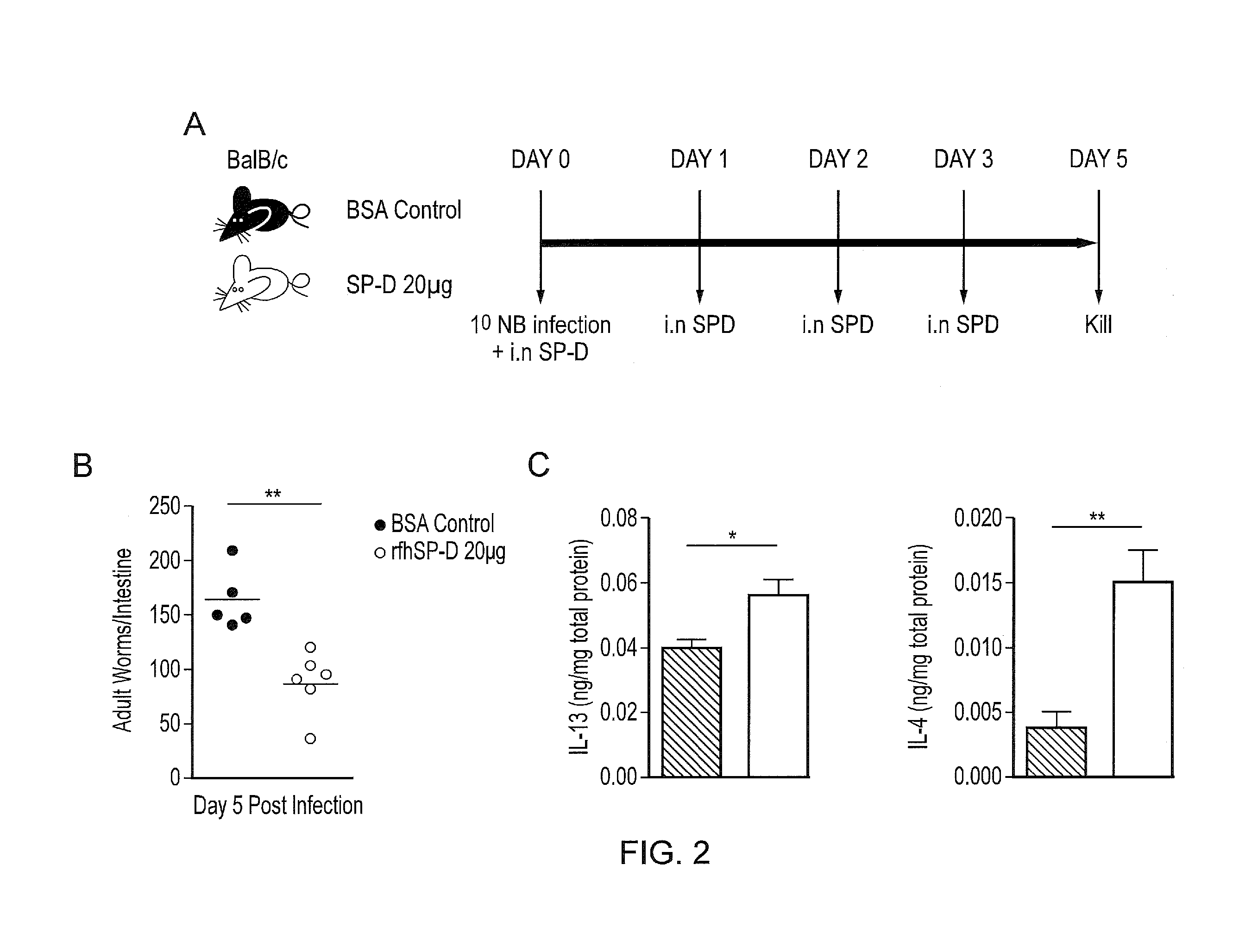
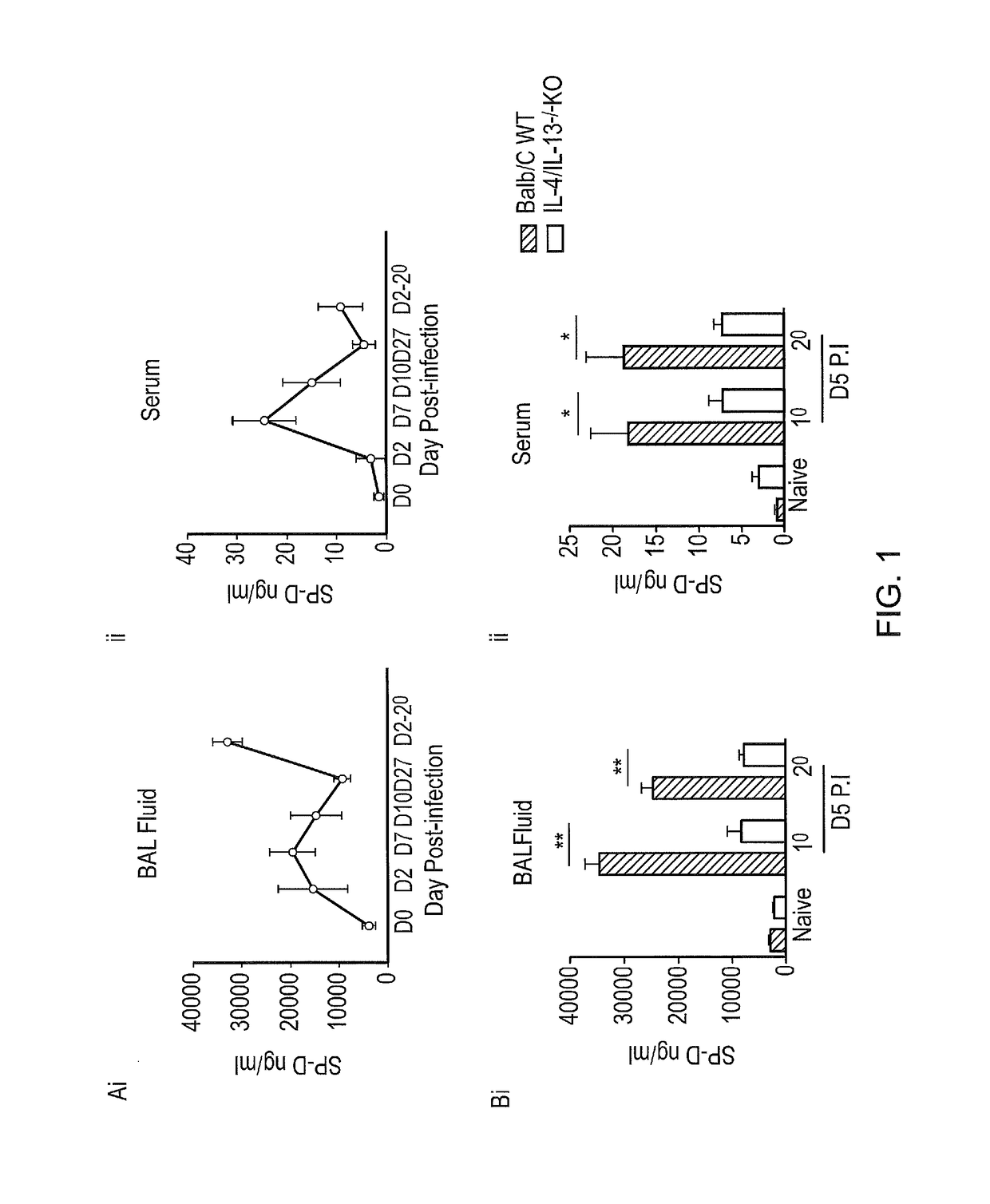
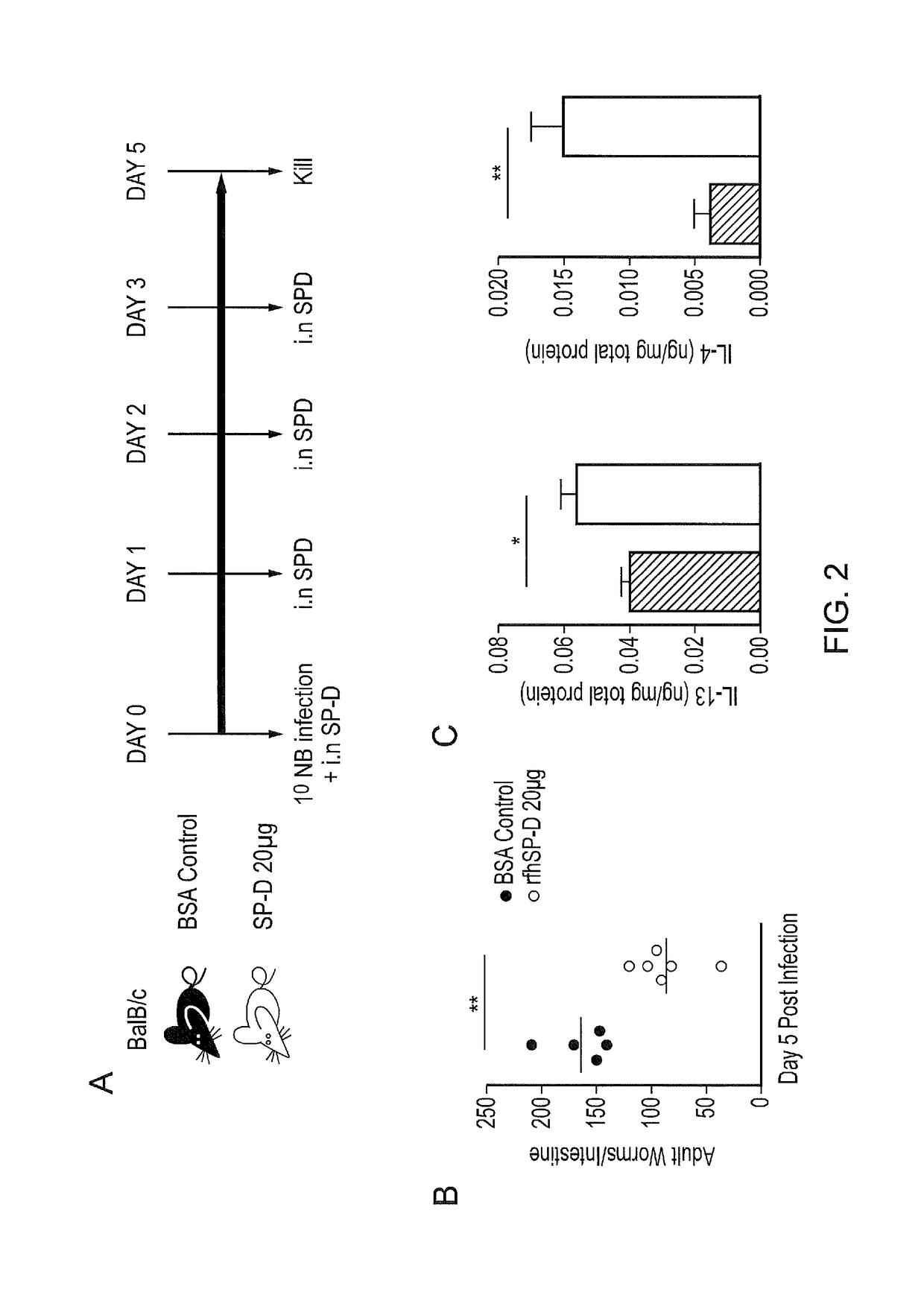
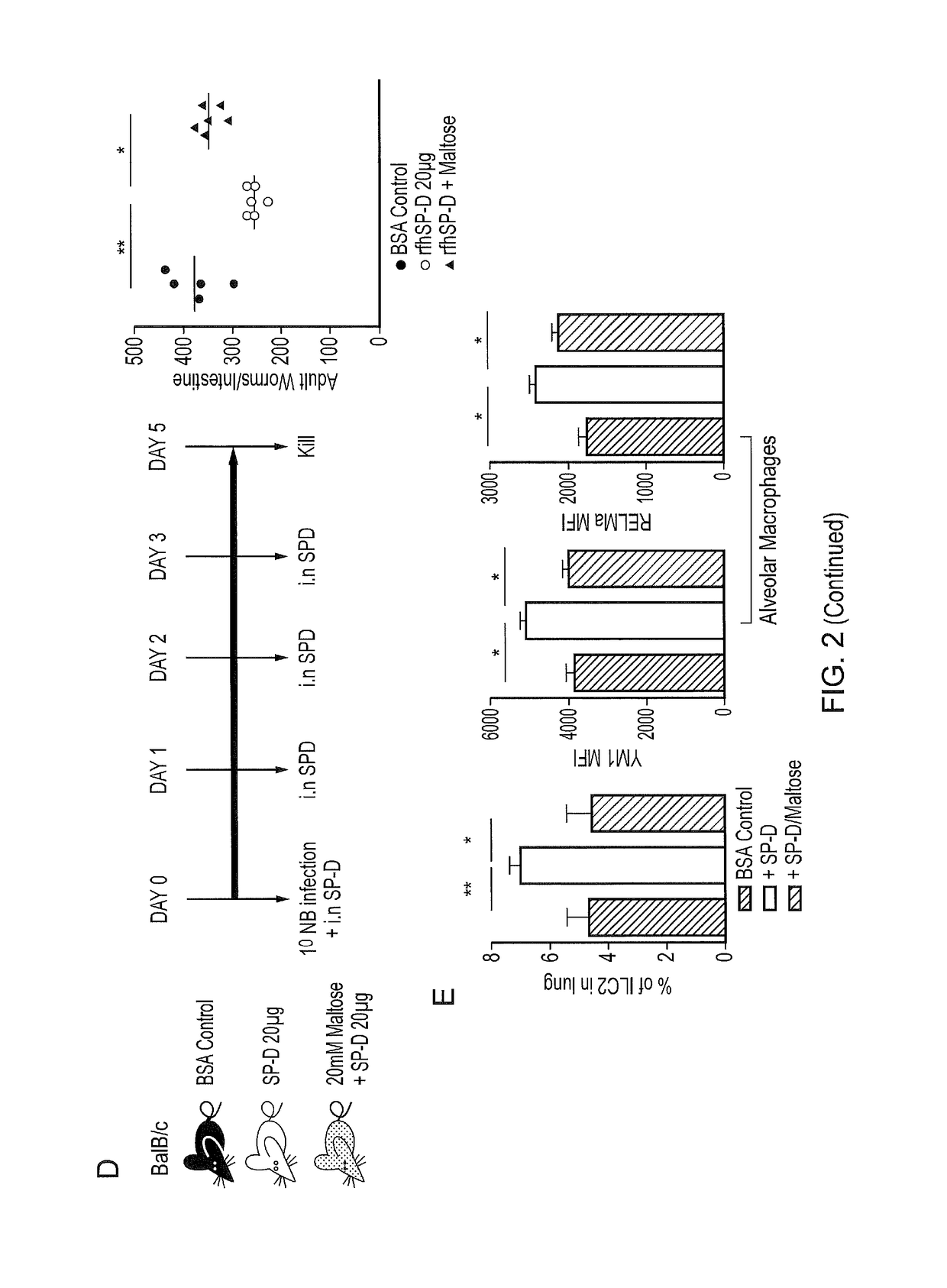
InactiveUS20170056481A1Reduces parasite burdenEnhances alternative activationPeptide/protein ingredientsAlveolar/pulmonary surfactant peptidesSurfactant protein AAllergy
The present invention relates to Surfactant Protein D (SP-D) or nucleic acids encoding SP-D or variants thereof such as surfactant protein A or mannan binding lectin for use in the treatment and / or prevention of a parasitic infection. Methods for determining the presence of a parasitic infection by determining levels of SP-D in a sample are also disclosed. Also disclosed are helminths for treating allergy, inflammation or infection.
Owner:UNIVERSITY OF CAPE TOWN +1
Colorectal cancer screening examination and early detection method
PendingUS20220214345A1Contributes immensely to the global burden of cancersReduce incidenceDisease diagnosisDiseaseBiomarker panel
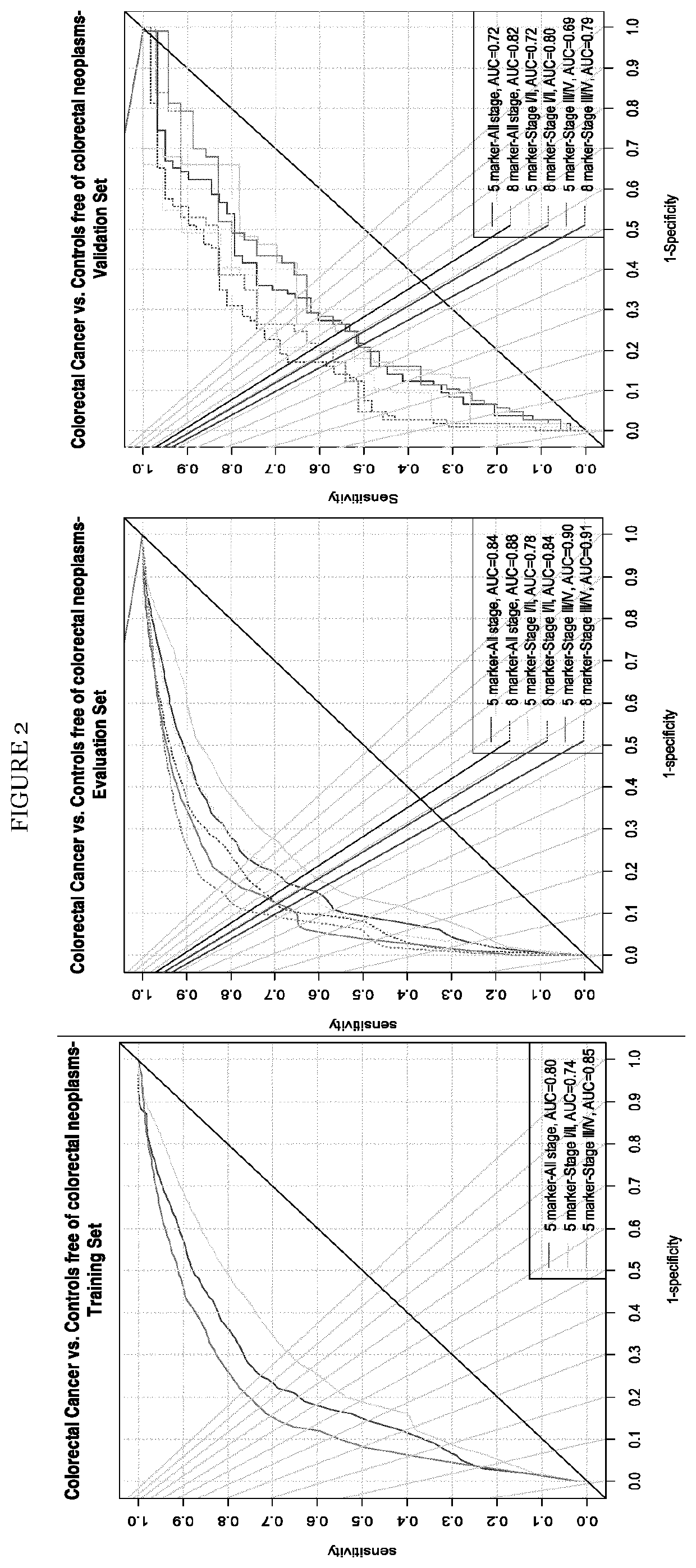
The present invention pertains to a new method for the diagnosis, prognosis, stratification and / or monitoring of a therapy, of cancer, preferably colorectal cancer (CRC), in a subject. The method is based on the determination of the level of a panel of least one, preferably 3, 4 and most preferably at least 5, protein biomarker selected from the group consisting of the protein biomarkers Amphiregulin (AREG), Carcinoembryonic antigen (CEA), Insulin like growth factor binding protein 2 (IGFBP2), Keratin, type I cytoskeletal 19 (KRT19), Mannan binding lectin serine protease 1 (MASP1), Osteopontin (OPN), Serum paraoxonase lactonase 3 (PON3) and Transferrin receptor protein 1 (TR), in the biological sample obtained from the subject. The new biomarker panel of the invention allows diagnosing and even stratifying various cancer diseases. Furthermore, provided are diagnostic kits for performing the non-invasive methods of the invention. Since the biomarker panel of the invention provides a statistically robust method independent of the protein detection technology used, and considering that the biomarker panel of the invention is detected in plasma samples of the subjects, the invention provides an early detection screening examination that may be applied to a larger population.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
Anti-mannan-binding lectin monoclonal antibodies and kit
InactiveCN103614341AStrong specificityHigh sensitivityImmunoglobulins against animals/humansMicroorganism based processesEpitopeHybridoma cell
Provided are anti-mannan-binding lectin monoclonal antibodies and a kit. Hybridoma cell strains comprise hybridoma cell strains used for coating the anti-mannan-binding lectin monoclonal antibodies, and with the culture number being CGMCC No. 0001, and also comprise hybridoma cell strains used for labeling the anti-mannan-binding lectin monoclonal antibodies, and the culture number being CGMCC No. 0002. The hybridoma cell strains used for coating the anti-mannan-binding lectin monoclonal antibodies and the hybridoma cell strains used for labeling the anti-mannan-binding lectin monoclonal antibodies have different binding epitopes. The invention aims to provide anti-mannan-binding lectin monoclonal antibodies and a kit with strong specificity, high sensitivity, wide detection range and accurate and reliable detection results.
Owner:杨旸
Modified-dextrans for use in optical glucose assays
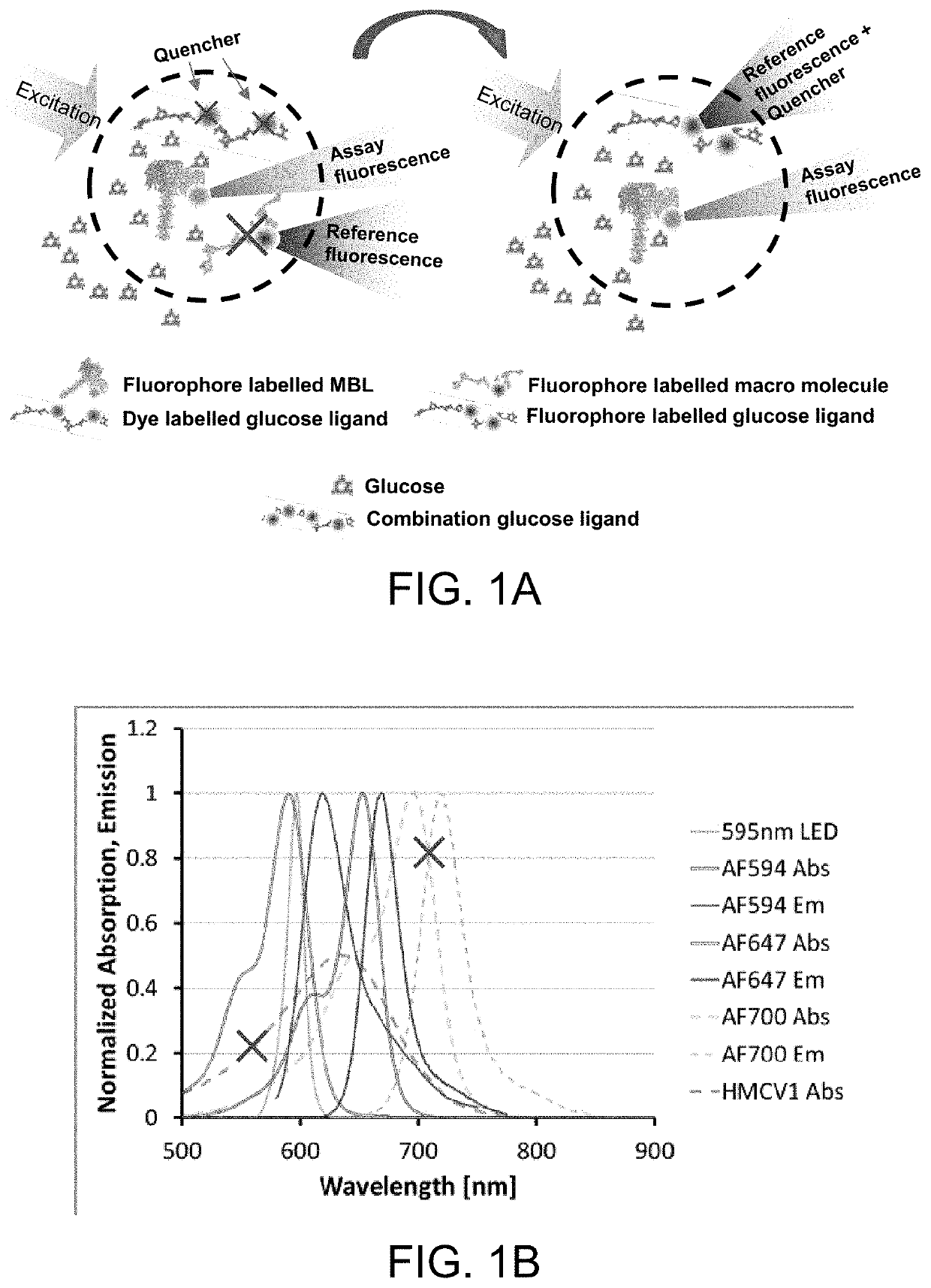
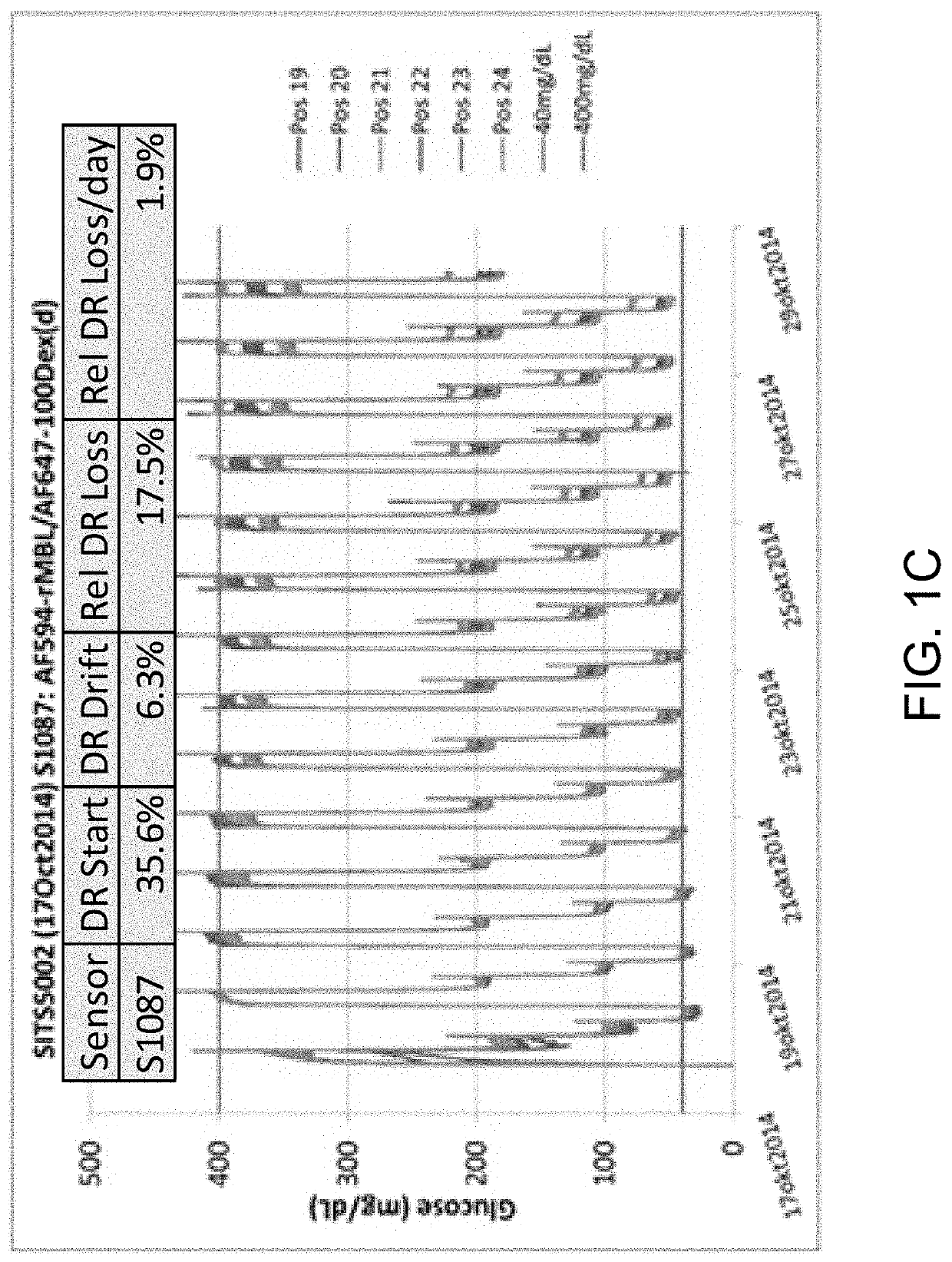
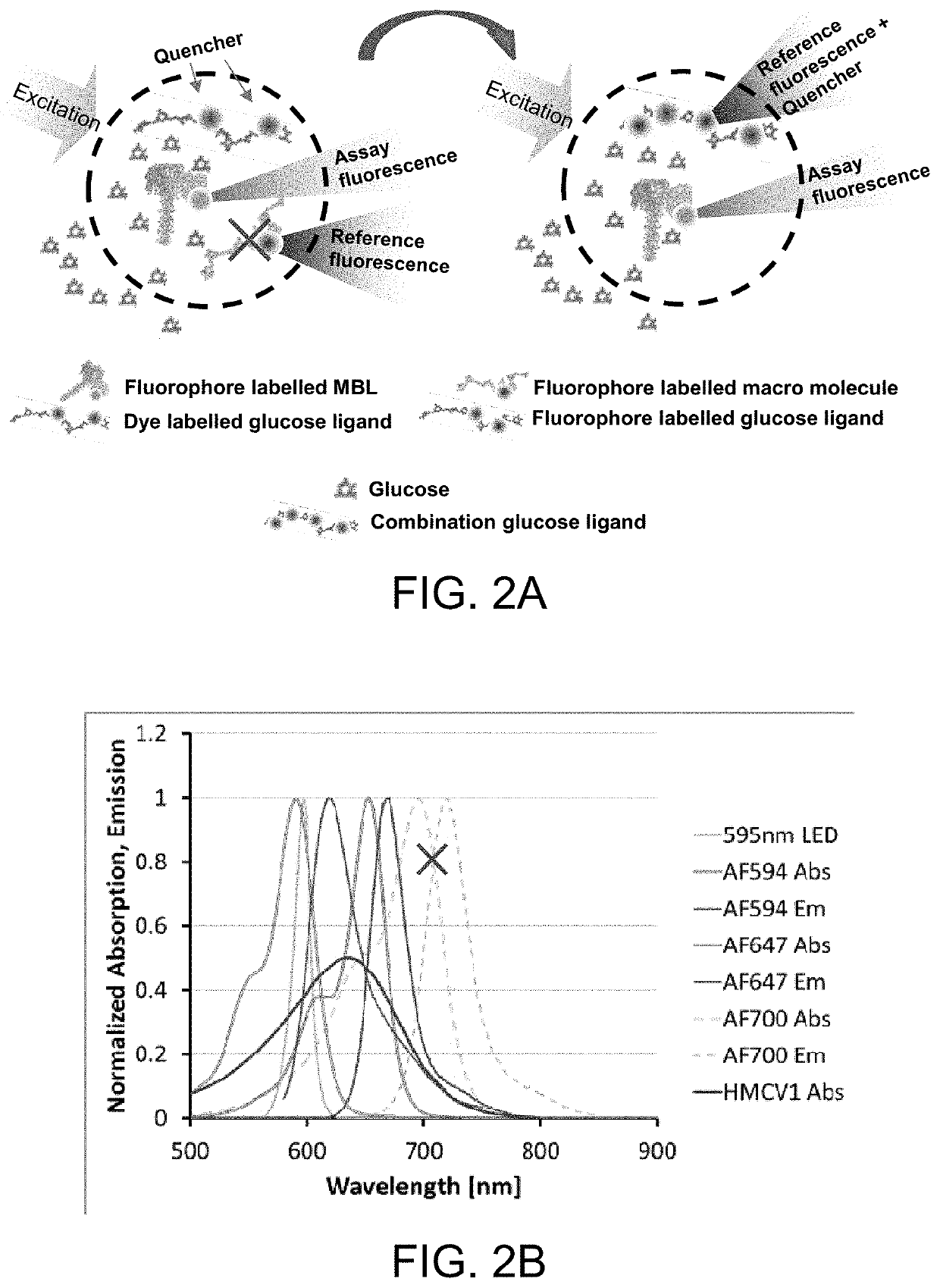
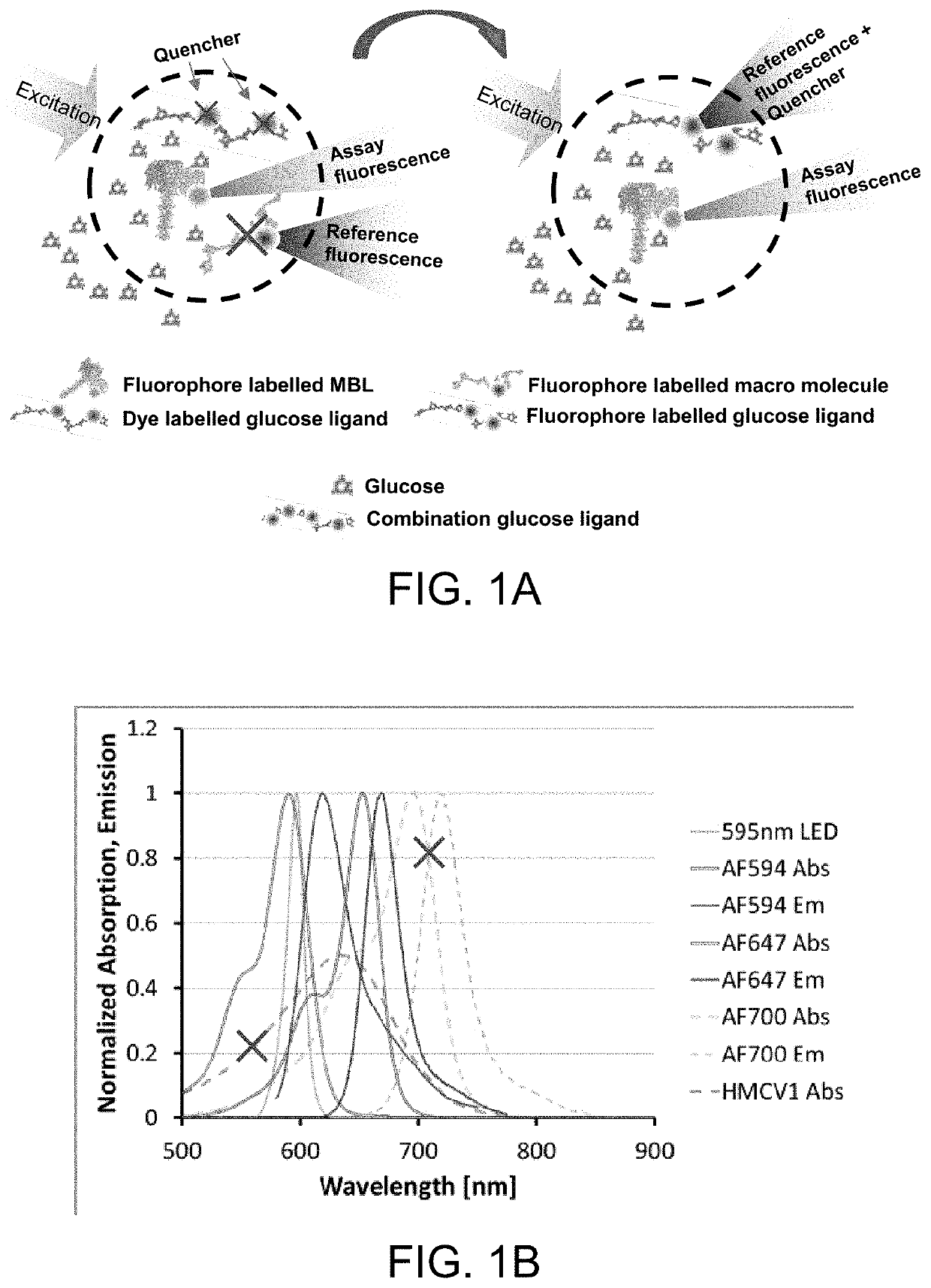
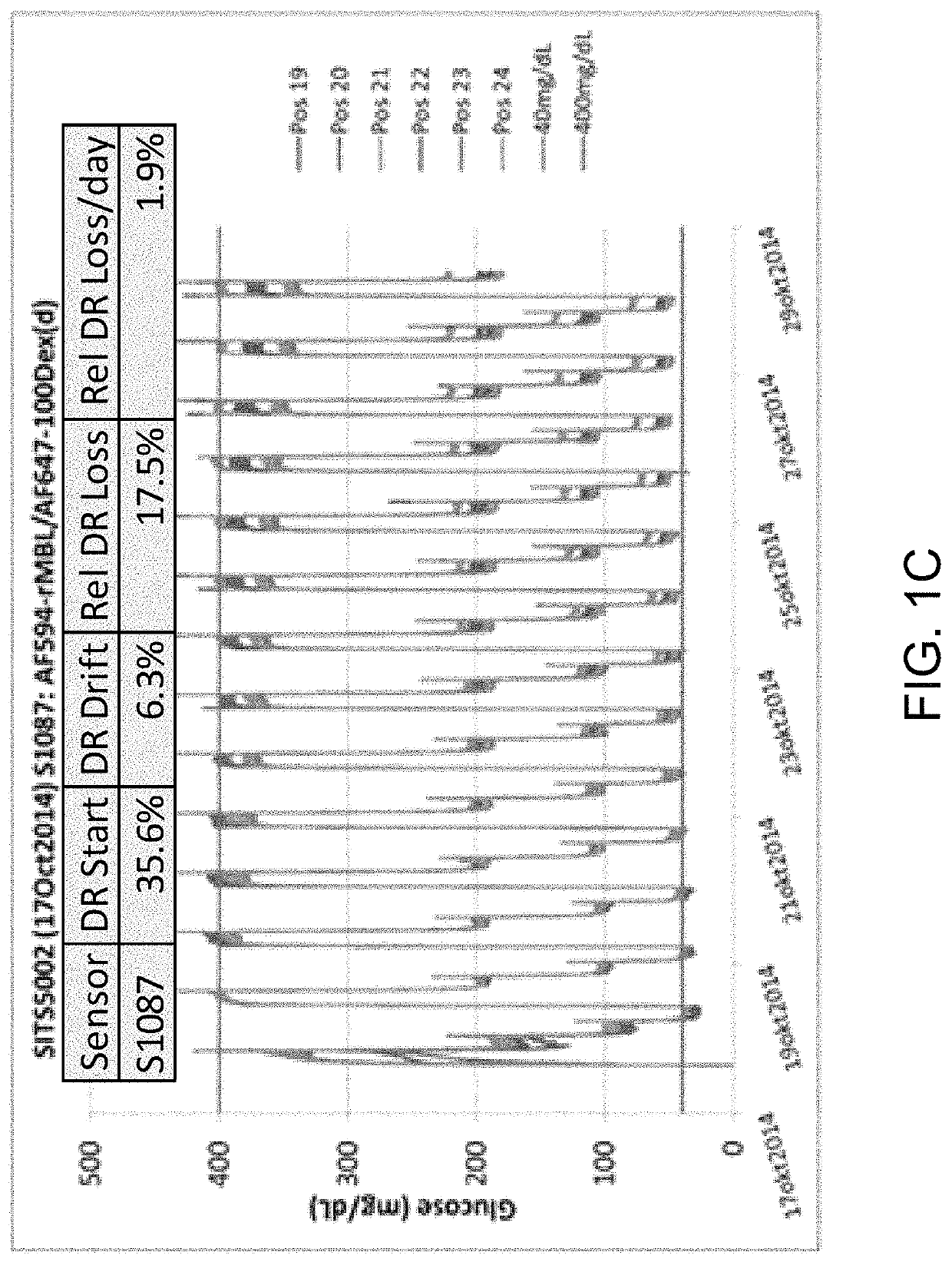
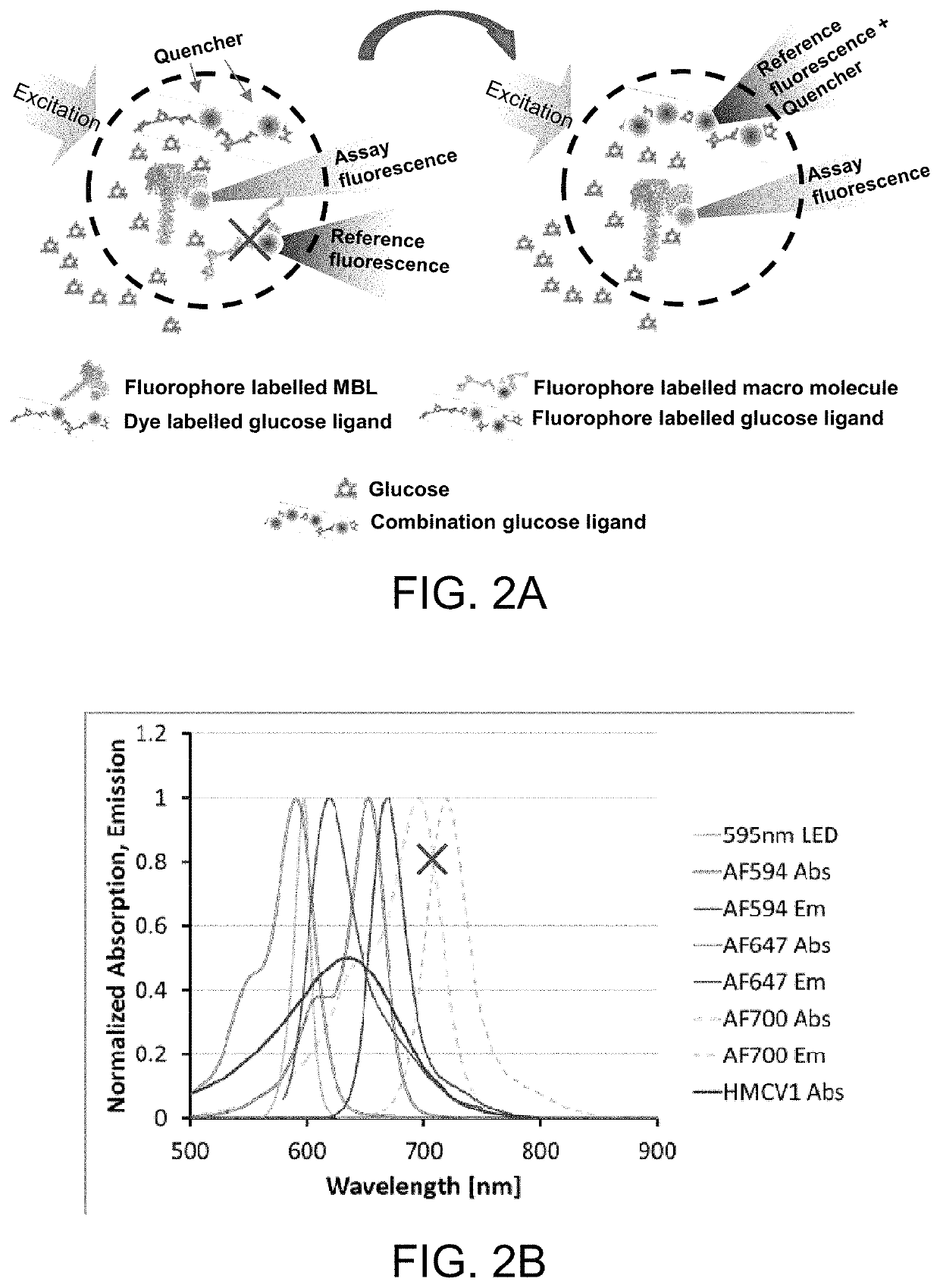
ActiveUS10543288B2Improve stabilityOptimized hydrophilic-hydrophobic balanceBiological material analysisBiological testingAssayFluorophore
The invention is directed to a competitive glucose binding affinity assay comprising a glucose receptor (typically mannan binding lectin) labeled with an assay fluorophore and a modified glucose analog (typically dextran) labeled with a reference fluorophore. In certain embodiments, the glucose analog is dextran and is coupled to both a reference fluorophore and a quencher dye (e.g. hexamethoxy crystalviolet-1). Optionally the reference fluorophore is blue shifted relative to the assay fluorophore.
Owner:MEDTRONIC MIMIMED INC
Application of mbl in the preparation of drugs for preventing or treating effector T-cell-induced diseases
ActiveCN107050436BEnhanced inhibitory effectRich sourcesAntibacterial agentsPeptide/protein ingredientsImmunologic disordersAutoimmune condition
The invention discloses the application of MBL in the preparation of drugs for preventing or treating effector T cell-induced diseases, and belongs to the technical field of new applications of mannan-binding lectins. The gist of the technical solution of the present invention is: the application of MBL in the preparation of drugs for the prevention or treatment of infectious diseases, autoimmune diseases or / and allergic diseases caused by effector T cells, wherein MBL inhibits the proliferation of effector T cells by enhancing iTreg cells achieve the efficacy of the drug. The present invention obtains the new factor that regulates Tregs cell inhibitory function through research, namely MBL directly strengthens Tregs cell to effector T cell proliferation inhibitory effect; The present invention highlights a kind of new function of MBL, not only plays an important anti-inflammatory role in innate immunity Infection, and in the intervention of Tregs cell function, it may be a target for the treatment of various autoimmune and inflammatory diseases.
Owner:XINXIANG MEDICAL UNIV
Methods and preparations for curing critically ill patients
InactiveUS20090181882A1High riskReduce mortalityAntibacterial agentsPeptide/protein ingredientsMedical wardCritically ill
The present invention pertains to the use of a blood mannan-binding lectin (MBL) regulator for the manufacture of a life saving drug to treat or cure a critically ill patient. It further involves the use of measurements of MBL to predict mortality in critically ill ICU patients. One further aspect of present invention is to the use of monomers and oligomers of MBL in prophylactic and / or curative treatment of patients admitted to intensive care units (ICUs).
Owner:K U LEUVEN RES & DEV +2
Novel indications of mannan-binding lectin (MBL) in the treatment of immunocompromised individuals
The present invention relates to at least one mannan-binding lectin (MBL) subunit, or at least one mannan-binding lectin (MBL) oligomer comprising at least one mannan-binding lectin. Use of a composition of (MBL) subunit) in the preparation of a medicament for preventing and / or treating infections. In particular, the present invention relates to the prevention and / or treatment of infections in immunocompromised individuals; and / or in immunocompromised susceptible individuals due to medical treatments. The present invention is particularly suitable for the prevention and / or treatment of infections in neutropenic patients and / or patients receiving or about to receive chemotherapy or similar treatments. Such individuals may be treated regardless of their serum MBL levels, but prophylaxis and / or treatment has been shown to be advantageous, particularly in patients with serum MBL levels between 50 ng / ml and 500 ng / ml.
Owner:斯蒂芬·蒂尔 +1
Using a blue-shifted reference dye in an optical glucose assay
ActiveUS10792378B2Improve stabilityOptimized hydrophilic-hydrophobic balanceBiological material analysisBiological testingAssayFluorophore
Owner:MEDTRONIC MIMIMED INC
Mannan-binding lectin (MBL) treatment of infections in individuals treated with TNF-alphainhibitors
InactiveUS7387993B2Improve adhesionFacilitated releaseAntibacterial agentsBiocideCurative treatmentOligomer
Owner:ENZON PHARM INC
Treating infection
InactiveUS10086050B2Reduces parasite burdenEnhances alternative activationConnective tissue peptidesPeptide/protein ingredientsSurfactant protein AAllergy
The present invention relates to Surfactant Protein D (SP-D) or nucleic acids encoding SP-D or variants thereof such as surfactant protein A or mannan binding lectin for use in the treatment and / or prevention of a parasitic infection. Methods for determining the presence of a parasitic infection by determining levels of SP-D in a sample are also disclosed. Also disclosed are helminths for treating allergy, inflammation or infection.
Owner:UNIVERSITY OF CAPE TOWN +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com