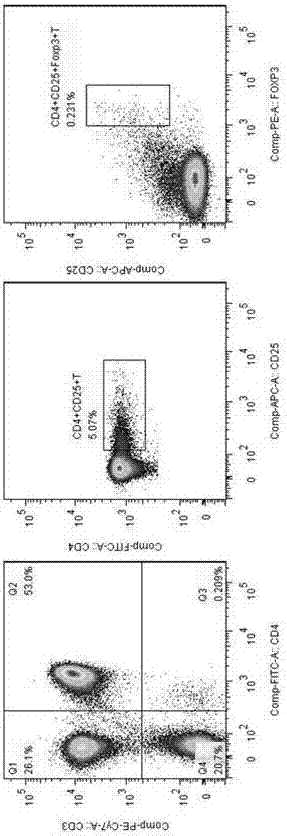
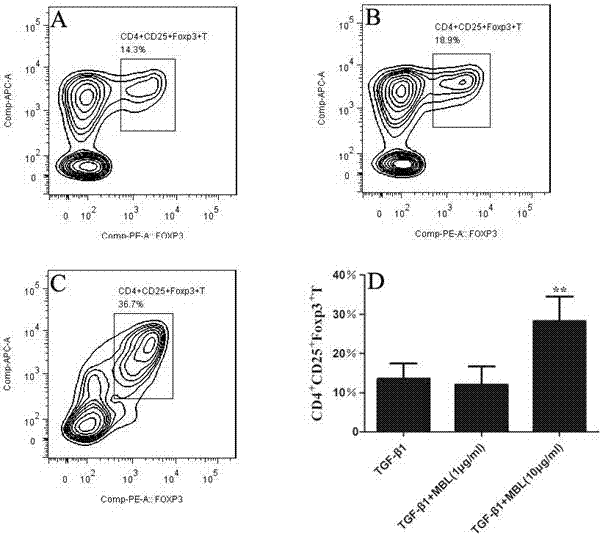
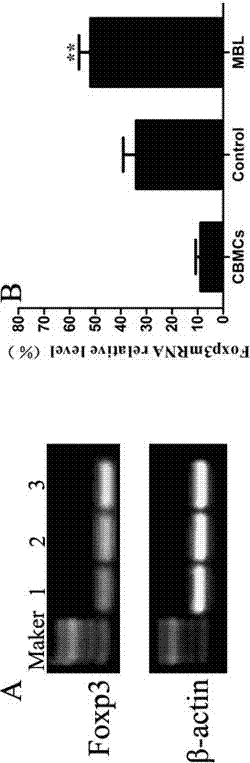
Application of MBL in preparing drug for preventing or treating disease taking Tregs as target spot
A disease, drug technology, applied in the field of new uses of mannan-binding lectin, to achieve the effect of enriching the source of umbilical cord
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Experimental Materials
[0030] 1. The source of cells used in the experiment
[0031] Neonatal umbilical cord blood was collected from the Third Affiliated Hospital of Xinxiang Medical College and the Department of Obstetrics and Gynecology of Xinxiang First People's Hospital of full-term healthy newborns. Immediately after the fetus was delivered, blood was collected from the umbilical vein of the placenta and placed in a blood collection bag containing 28 mL of preservation solution. All blood samples were delivered to the laboratory within 4 hours after sampling.
[0032] 2. Main reagents
[0033]Medium RPMI1640, fetal bovine serum (FBS), and 10×PBS solution were all purchased from Thermo Fisher Scientific.
[0034] Lymphocyte separation medium (Ficoll-Paque PLUS), which is a sterile endotoxin (<0.12 EU / mL) test solution of FicollTMPM400 and sodium diatrizoate with a density of 1.077g / mL, was purchased from GE Healthcare Life Sciences.
[0035] Anti-human CD3 mon...
Embodiment 2
[0118] Experimental Materials
[0119] 1. The source of cells used in the experiment
[0120] Reference example 1
[0121] 2. Main reagents
[0122] Reference example 1
[0123] 3. Main instruments and equipment
[0124] Reference example 1
[0125] 4. Preparation of common reagents
[0126] Reference example 1
[0127] experimental method
[0128] 1. Preparation of umbilical cord blood mononuclear cells
[0129] Reference example 1
[0130] 2. Coated with anti-CD3mAb
[0131] Reference example 1
[0132] 3. Magnetic separation of CD4+CD25-T cells and CD4+CD25+T cells
[0133] sample preparation
[0134] Peripheral mononuclear cells were separated by density gradient centrifugation. To remove platelets, cells were resuspended in buffer and centrifuged at 200×g for 10-15 min. The supernatant was carefully aspirated and washed repeatedly.
[0135] Magnetic labeling of non-CD4+ T cells
[0136] The whole process should be completed quickly, and the cells should be k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com