Lysosome-targeted hypochlorite ion fluorescent probe and application thereof
A fluorescent probe and hypochlorite technology, applied in the field of analytical chemistry, can solve the problems of inability to detect hypochlorite, low sensitivity, complex synthesis, etc., and achieve novel probe structure, strong anti-interference ability, and simple post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Preparation of target probe compound N16: 1) Synthesis of compound N16-1: Dissolve 1g (3.6mmol) of 4-bromo-1,8-naphthalimide in ethanol, add 1g (7.2mmol) of morpholine, reflow. After the reaction was completed, the reaction solution was cooled at room temperature, the solid was filtered off, and purified by column chromatography, which was the target product N16-1 with a yield of 85%; 2) Synthesis of compound N16: 388 mg (1 mmol) N16-1 and 276 mg (2 mmol) ) Potassium carbonate was dissolved in 5 mL of DMF, 140 mg (2 mmol) of sodium methyl mercaptide was added, and reacted at a constant temperature of 80° C. for 2 h under the protection of nitrogen. After the reaction, the reaction solution was cooled to room temperature and poured into 40mL of water. A solid was produced, which was filtered and purified by chromatography to obtain N16 with a yield of 88%.
Embodiment 2
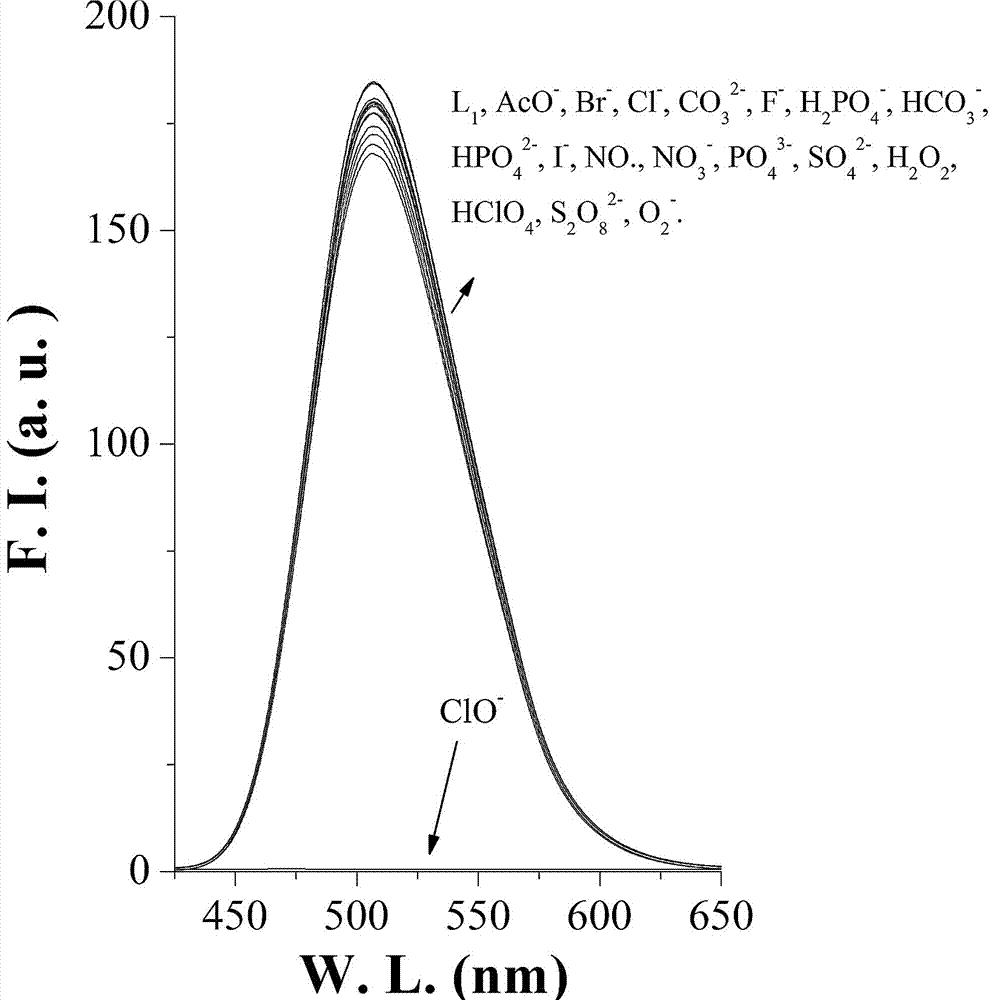
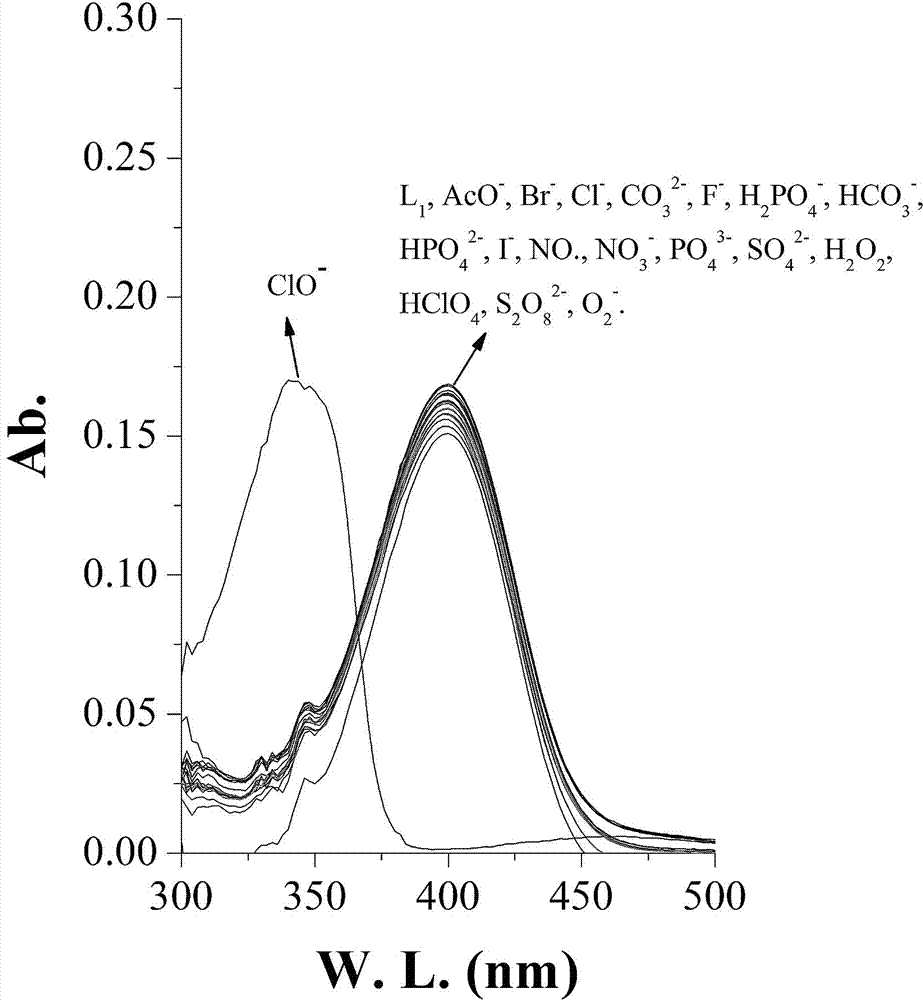
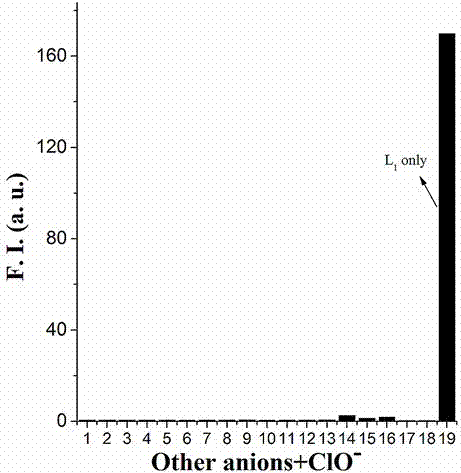
[0022] Example 2. N16 to ClO - Fluorescence selectivity experiment of 1: Take the N16 hypochlorite fluorescent probe prepared in Example 1 and dissolve it in DMF to make a 1mmol / L stock solution. Take 30 μL of the stock solution and add it to 3 mL of PB buffer solution (pH=7.4), and then add 30 μL of different anions and cations at a concentration of 100 μM, ClO - The addition of ions completely quenched the fluorescence. The excitation wavelength is 405nm, and the emission wavelength is 506nm. The ordinate indicates the fluorescence intensity, and the abscissa indicates the wavelength; N16 is to ClO - UV selectivity experiment: take 30 μL of the fluorescent probe stock solution in Example 2 and add it to 3 mL of PB buffer solution (pH=7.4), and add 30 μL of different anions and cations with a concentration of 100 μM respectively. The visible absorption spectrum. ClO - The absorbance at 405nm decreased significantly, and the absorbance at 350nm increased significantly. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com