High-purity P1,P4-bis(uridine-5'-tetraphosphoric acid) salt preparation method
A technology of tetraphosphoric acid and uridine, applied in the field of P1, can solve problems such as difficult to reduce medicinal requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: UMP-2Bu 3 NH preparation
[0070] Add 208.5g (0.566g) of disodium salt of UMP to 1.25L of water and stir to dissolve, add the aqueous solution to the cation exchange resin (hydrogen type), soak for 2 hours, rinse with water until no UMP flows out, combine the effluents, and add 214.8g of tributylamine (1.159mol), stirred at room temperature for 1h, concentrated to dryness under reduced pressure at 60°C, concentrated to dryness with 500ml of dioxane with azeotropic water, dissolved in 2.5L of N,N dimethylformamide, and stored within 0°C for later use.
Embodiment 2
[0071] Example 2: UTP-3Bu 3 NH preparation
[0072] Add 289.4g (0.526mol) of trisodium salt of UTP to 1.25L of water and stir to dissolve, add the aqueous solution to the cation exchange resin (hydrogen type), rinse with water until no UTP flows out, combine the effluents, and add 394.8g (2.13 mol), stirred at room temperature for 1 h, concentrated to dryness under reduced pressure at 50°C, concentrated to dryness with 500ml of dioxane with azeotropic water, dissolved in 2.5L of N,N dimethylformamide, and stored within 0°C for future use.
Embodiment 3-9
[0073] Example 3-9, P 1 , P 4 - Preparation of di(uridine-5'-tetraphosphate)
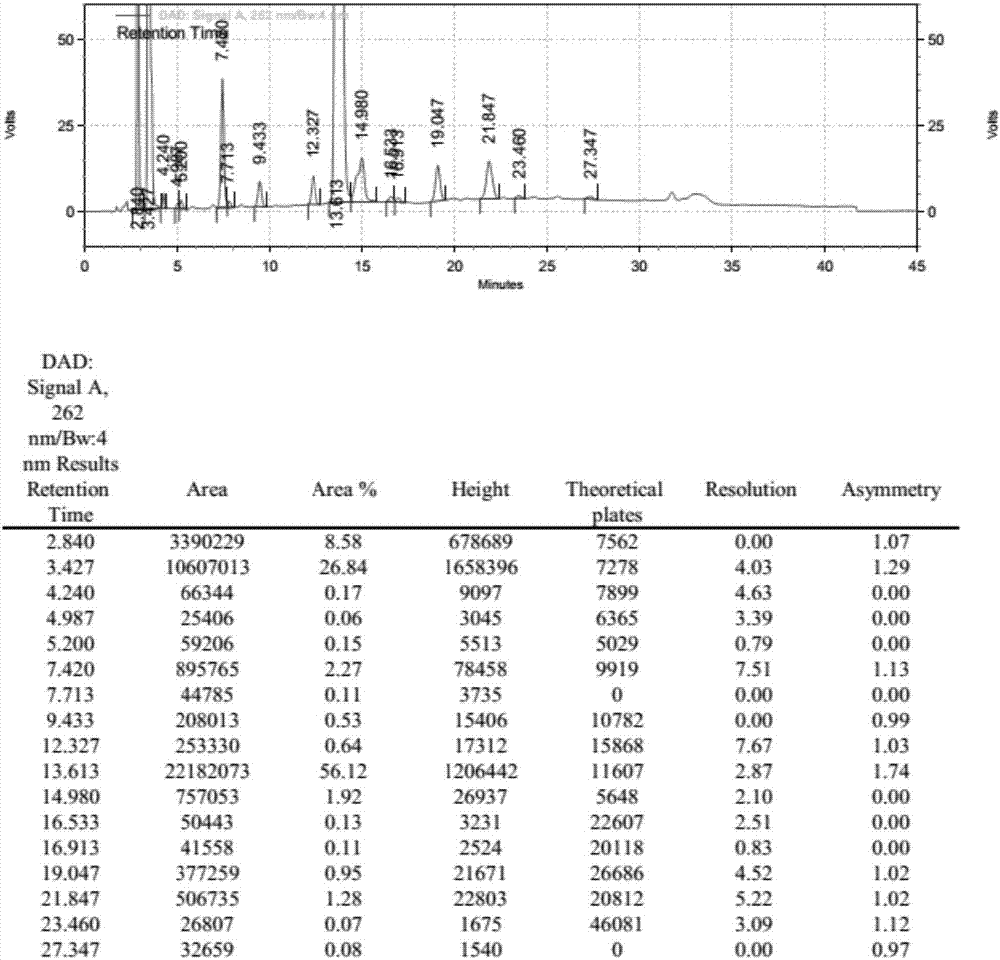
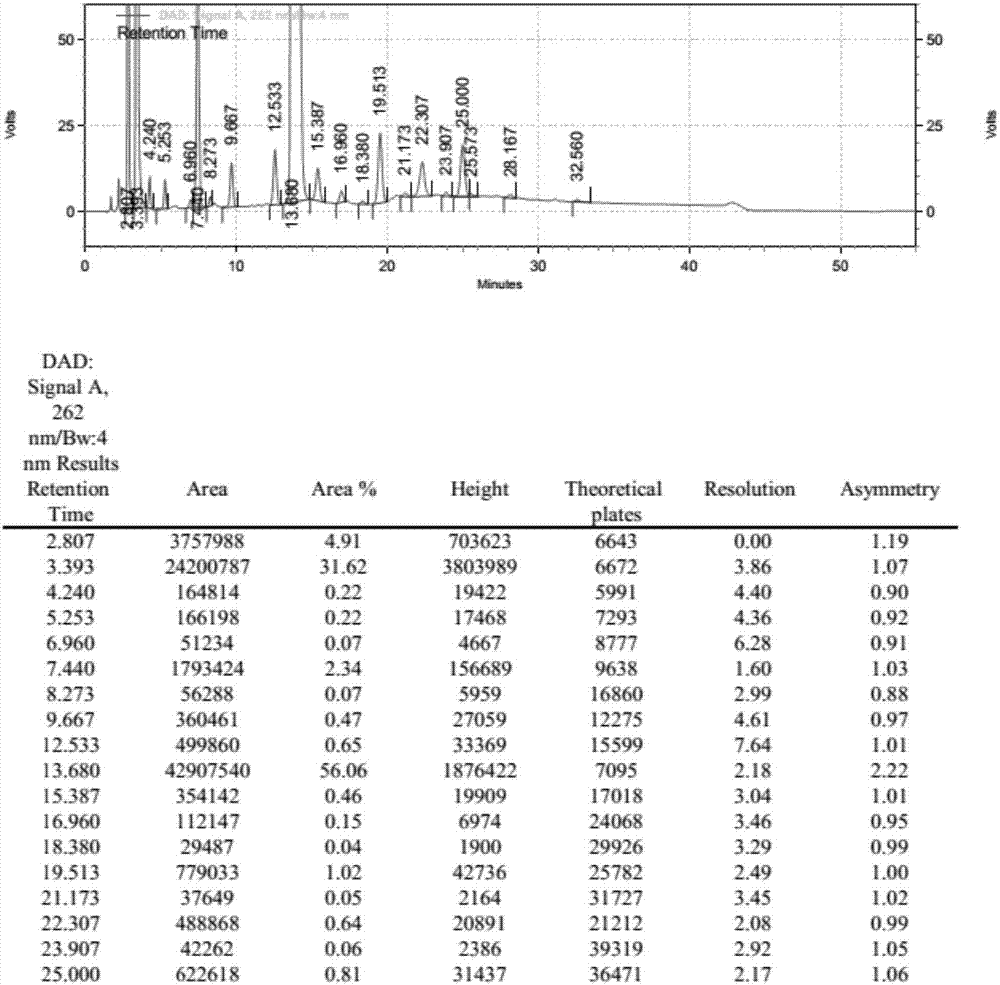
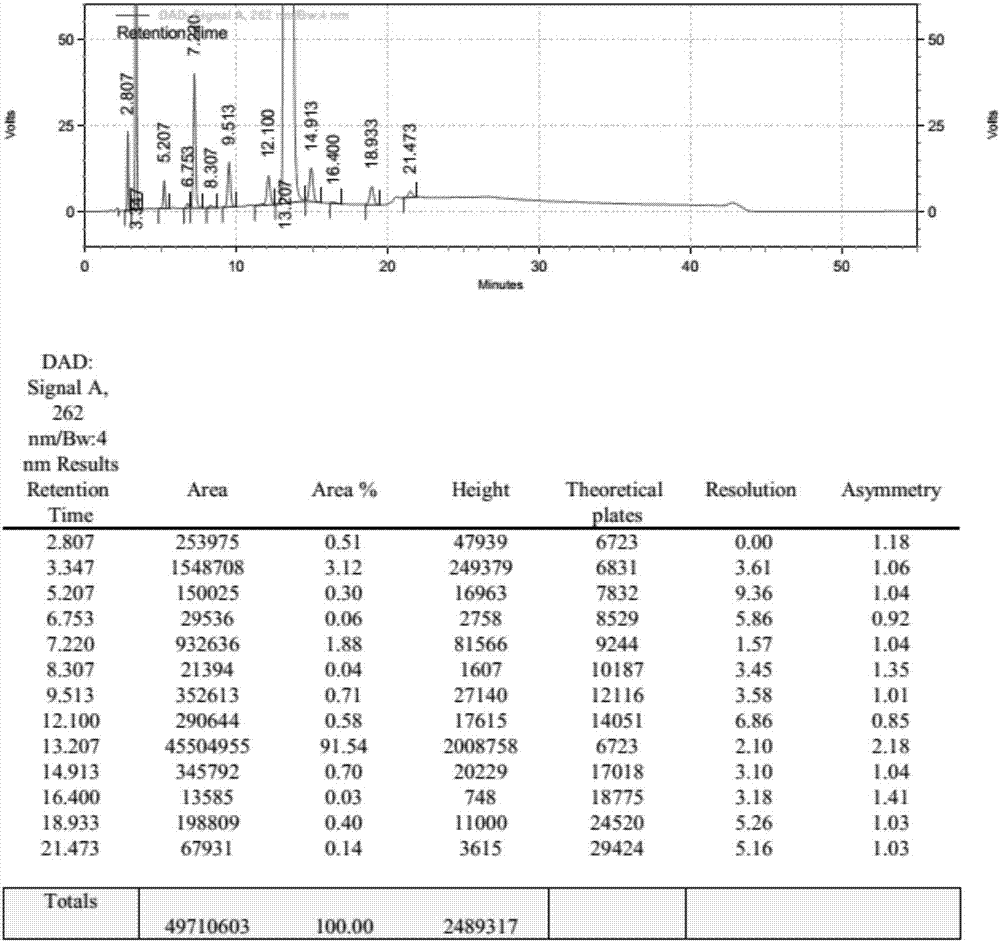
[0074] UTP-3Bu 3 NH N, N dimethylformamide solution 25.0ml (5.0mmol), 18.0ml (3.6mmol), 13.5ml (2.7mmol), 10.8ml (2.16mmol), 9.0ml (1.8mmol), 6.75ml (1.35 mmol), 5.4ml (1.08mmol), add N,N diisopropylcarbodiimide 1.04g under stirring, react at 25℃ for 2h, add UMP-2Bu 3 NH N, N dimethylformamide solution 25ml (5.4mmol) and 1.36g (10.0mmol) zinc chloride, reacted at 25°C for 3h, after the reaction was complete, added 20ml of water to quench, concentrated to dryness under reduced pressure at 65°C, added 100ml of water was stirred and crystallized for 30min, filtered, and the filtrate was collected to obtain P 1 , P 4 - tributylamine salt of di(uridine-5'-tetraphosphate). HPLC detects purity, and the result is as follows:
[0075] Table 2: Change list of related substances under different ratios of UMP:UTP
[0076]
[0077] It can be clearly seen from the above table that with the continuous i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com