Difluorobithiophene-based donor-acceptor polymers for electronic and photonic applications
A conjugated polymer, D-A technology, applied in the field of new organic compounds, D-A conjugated polymers, can solve the problems of PSC performance deterioration and achieve the effects of life and efficiency improvement, good application prospects, and excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1 - Synthesis of monomers
[0130]
[0131] step 1: Preparation of 4,7-bis(4-(2-octyldodecyl)-2-thienyl)-2,1,3-benzothiadiazole (S3)
[0132] 3-(2-Octyldodecyl)thiophene (5.00 g, 13.7 mmol) was dissolved in 50 mL THF to obtain a solution, and the solution was cooled to -78° C. under nitrogen protection. Lithium diisopropylamide (2M, 8.3 mL, 16.6 mmol) was slowly added dropwise thereto, and the mixture was stirred at -78°C for 1 hour, then returned to 0°C and stirred for another 1 hour. The mixture was then cooled to -78°C and tri-n-butyltin chloride (6.50 g, 20 mmol) was added in one portion. The reaction mixture was brought to room temperature and stirred overnight. After adding KF aqueous solution, the organic phase was washed three times with water, and then washed with Na 2 SO 4 dry. The solvent was removed by rotary evaporation to obtain a yellow oily crude product, which was used directly without further purification. 2-(tri-n-butylstannyl)-4-...
Embodiment 2
[0138] Example 2 - Synthesis of polymers
[0139]
[0140] ffT2-TBTT can be synthesized by microwave reaction or conventional reaction. in N 2 In a protected glove box, monomer S4 (96.5mg, 0.095mmol), (3,3'-difluoro-[2,2'-dithiophene]-5,5'-diyl)bis(trimethyl stannane) (50.2mg, 0.095mmol), Pd 2 (dba) 3 (1.1mg, 0.002mmol) and P(o-tol) 3 (2.4 mg, 0.008 mmol) was added 1.6 mL of chlorobenzene. The reaction mixture was then sealed and heated at 145°C for 2 days (or microwaved at 160°C for 30 minutes). The mixture was cooled to room temperature, and 10 mL of toluene was added, followed by precipitation with methanol. The crude product was collected by filtration and extracted by Soxhlet (CH 2 Cl 2 , CHCl 3 and chlorobenzene) and repeated precipitation to purify. The solvent was removed by rotary evaporation, and the residue was dissolved in chlorobenzene and precipitated with methanol. The solid was collected by filtration and dried in vacuo to afford the polymer (89...
Embodiment 3
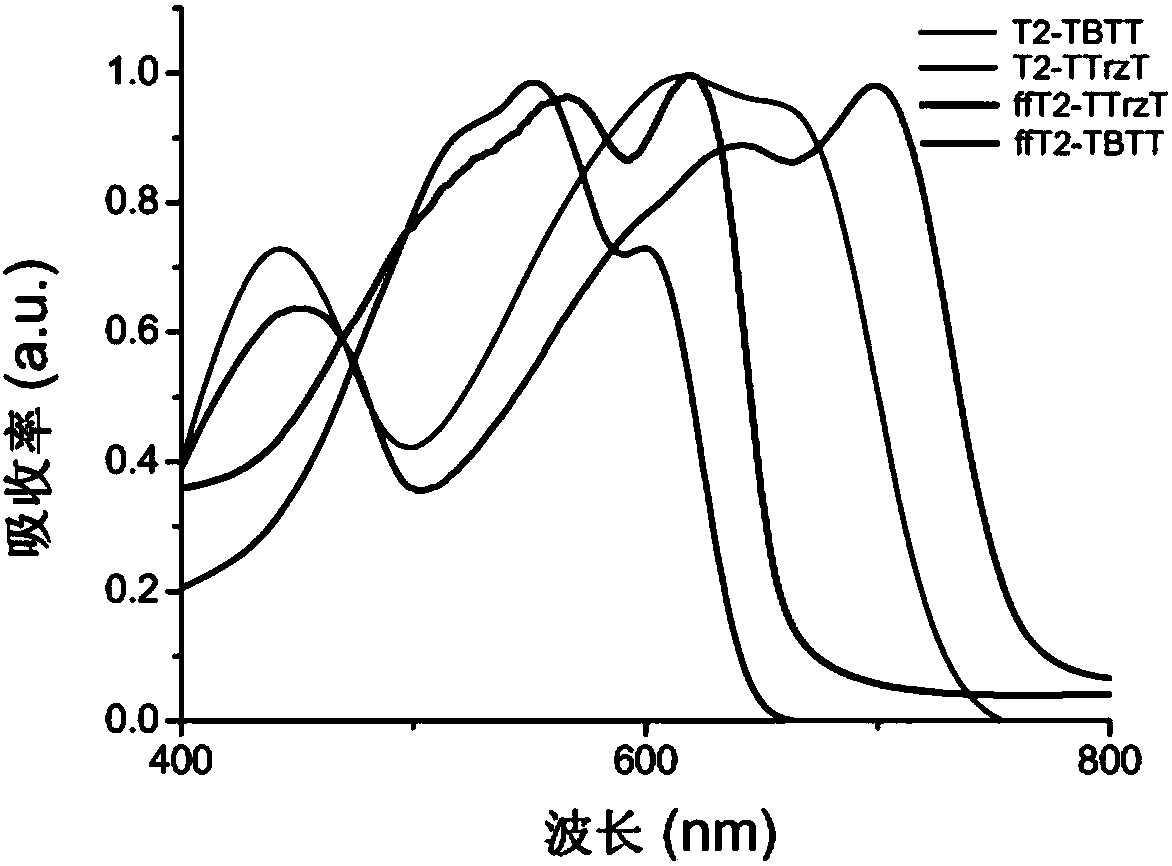
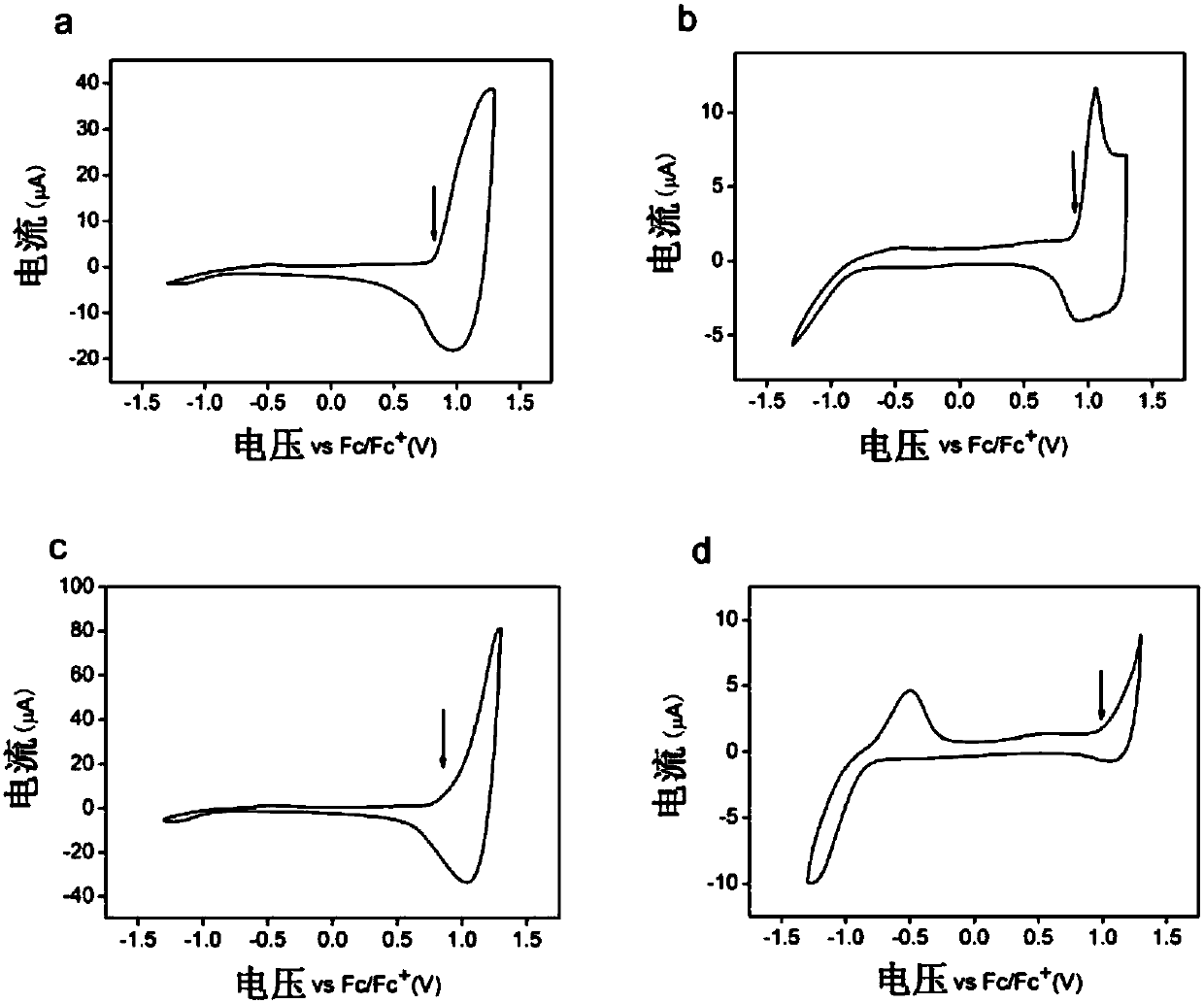
[0144] Example 3 - Characterization of polymers
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com