Nitrogen and boron co-doped carbon nano microspheres and preparation method thereof
A technology of carbon nano-microspheres and co-doping, which is applied in the direction of nano-carbon, etc., to achieve the effect of expanding the application field, regular shape and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Preparation of borate polymer microspheres:
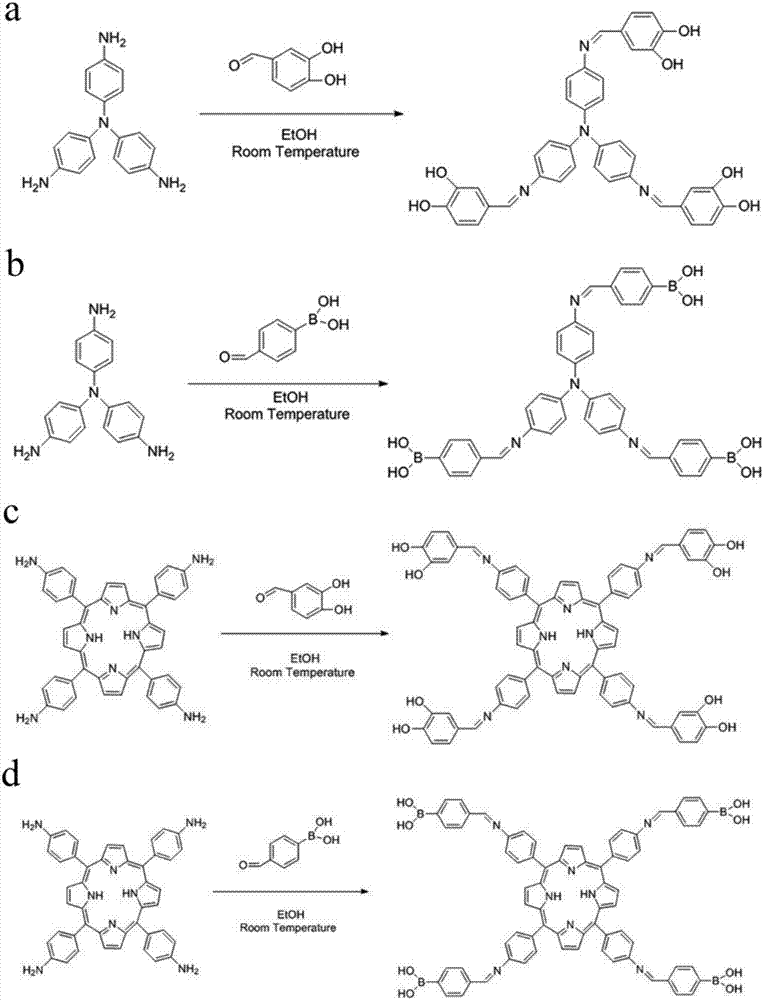
[0034] 1-1) Dissolve tris(4-aminophenyl)amine (0.145g, 0.5mmol) and 3,4-dihydroxybenzaldehyde (0.207g, 1.5mmol) in 20mL ethanol, at room temperature (eg 25°C) The reaction was stirred at 400 rpm for 24 h in the dark. For kinetic research, samples are taken at set time intervals for GPC and NMR testing. After the reaction is completed, the black solution obtained is the catechol group-containing monomer TC.
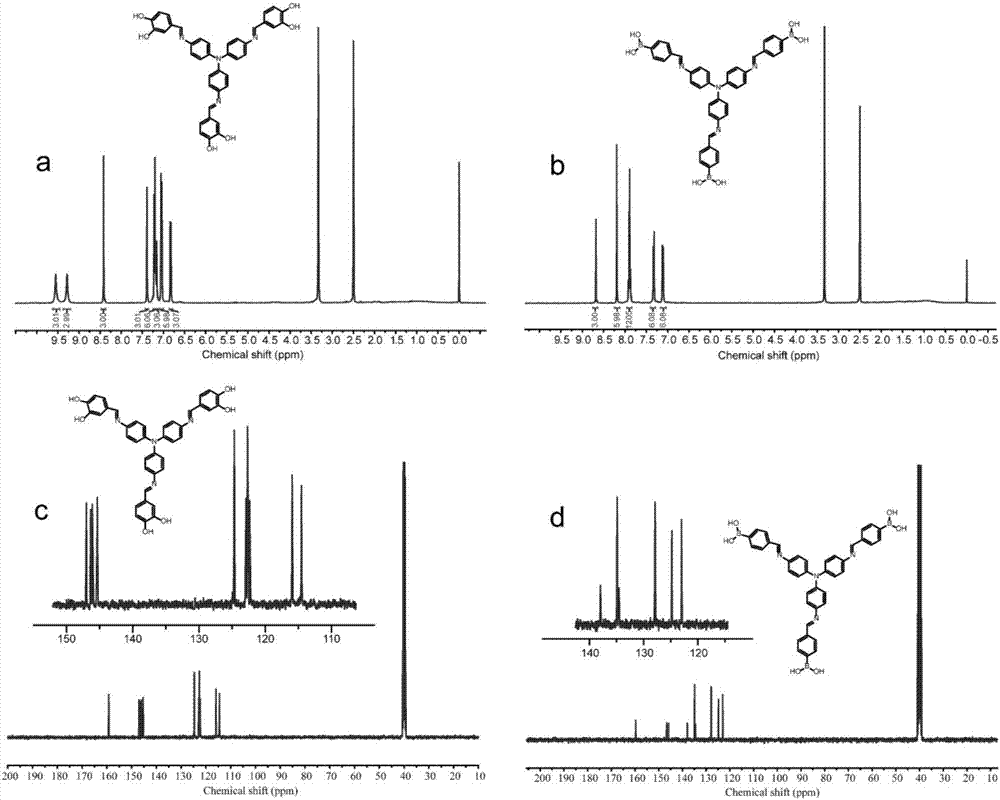
[0035] The synthetic reaction formula of this catechol group monomer TC is as follows figure 1 as shown in a. The proton nuclear magnetic resonance spectrum ( 1 H NMR) and carbon nuclear magnetic resonance ( 13 C NMR) results such as figure 2 a and figure 2 As shown in c, the solvent is deuterated dimethyl sulfoxide (DMSO-D 6 ). The peak positions in the hydrogen spectrum are assigned as follows: 9.70(s,3H), 9.55(s,3H), 8.61(s,3H), 7.39(d,3H), 7.21(d,6H), 7.16(d,3H), 7.05 (d, 6H), 6.83 (d, 3H). The peak p...
Embodiment 2
[0046] 1) Preparation of borate polymer microspheres:
[0047] 1-1) Dissolve 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (0.3374g, 0.5mmol) and 3,4-dihydroxybenzaldehyde (0.276g, 2mmol) in 20mL of ethanol , the reaction was stirred at 400 rpm for 24 h at room temperature (eg 25° C.) in the dark. For kinetic research, samples are taken at set time intervals for GPC and NMR testing. After the reaction is completed, the black solution obtained is the monomer PC containing catechol groups. The synthetic reaction formula of this catechol group monomer PC is as follows figure 1 as shown in c.
[0048] 1-2) Dissolve 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (0.3374g, 0.5mmol) and 4-formylphenylboronic acid (0.3g, 2 mmol) in 20mL of ethanol, The reaction was stirred at 400 rpm for 24 h at room temperature (eg, 25° C.) in the dark. For kinetic research, samples are taken at set time intervals for GPC and NMR testing. After the reaction is completed, the dark yellow solution obt...
Embodiment 3~4
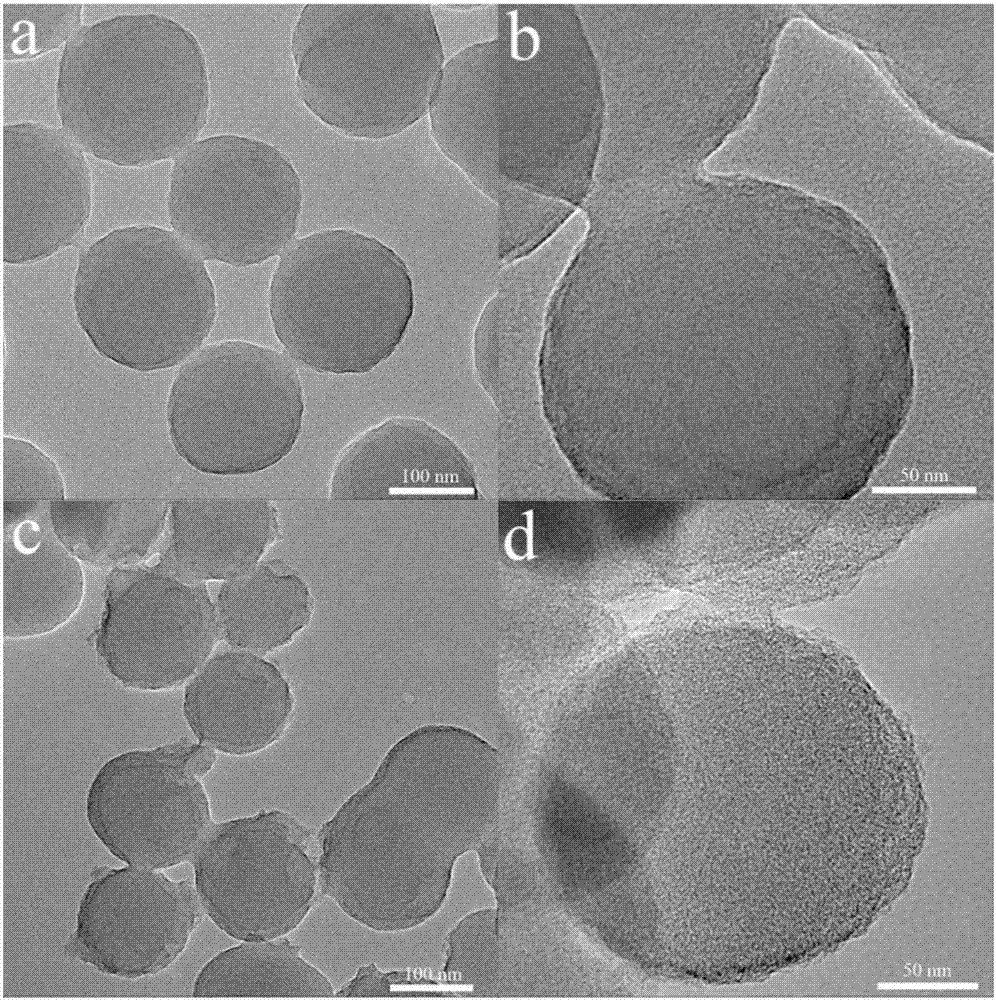
[0054] Embodiments 3 to 4: With reference to the process conditions of Example 1, in the step of preparing borate polymer microspheres, the ratio between the monomer containing catechol group and the monomer containing phenylboronic acid group was changed, and the synthesis was different. The morphology of borate polymer microspheres was used to prepare nitrogen and boron co-doped carbon nanospheres, as shown in Table 1.
[0055] Table 1 Reaction conditions for the synthesis of borate polymer microspheres with different morphologies in Examples 3-4
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com