Detection method of iodine ions in urine based on alcohol solvent-induced silver nanocluster fluorescence enhancement
A technology of silver nanoclusters and fluorescence enhancement, which is applied in the field of biomedical analysis and detection, can solve the problems of time-consuming sample pretreatment process, high environmental requirements, complex operation, etc., and achieve the effect of visual quick measurement, high sensitivity and enhanced fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] To prepare alcohol-induced fluorescence-enhanced silver nanoclusters (AgNCs) probes, the steps are as follows:
[0038] 1) Preparation of silver nanoclusters (AgNCs) materials:
[0039] a) At room temperature, add 125 μL of 20 mM silver nitrate (AgNO 3 ) Mix the aqueous solution with 150 μL of 50 mM glutathione (GSH), then add 5.0 mL of ultrapure water, stir and mix evenly, adjust the pH to 9-10 with 1.0 M NaOH aqueous solution to obtain solution A;
[0040] b) Combine 4.2 mg α-lipoic acid (LA) with 1.9 mg sodium borohydride (NaBH 4 ) Mix with 1 ml of ultrapure water, stir until LA is completely dissolved to obtain solution B, add 700 μl of solution B dropwise to solution A, stir for 20 min, let stand at room temperature for 1.5 h, then centrifuge After separation and purification, the silver nanocluster (AgNCs) material was prepared, and stored at 4°C and protected from light for later use;
[0041] 2) Preparation of silver nanoclusters (AgNCs) probes with alcohol-induced fluo...
Embodiment 2
[0044] Based on the alcohol solvent-induced silver nanocluster fluorescence enhancement method for detecting iodide ions in urine, the alcohol-induced fluorescence enhancement silver nanocluster probe prepared in Example 1 is used to determine iodide ions in urine (children’s morning urine), and the steps include:
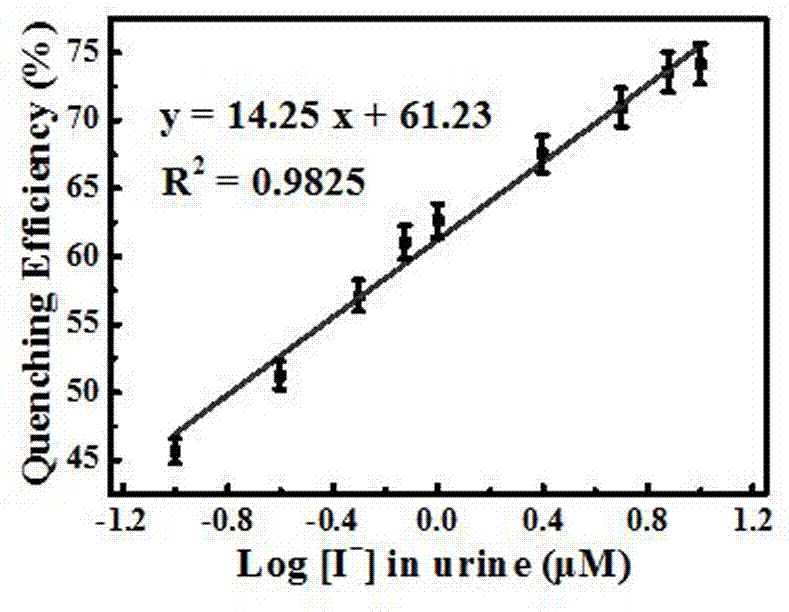
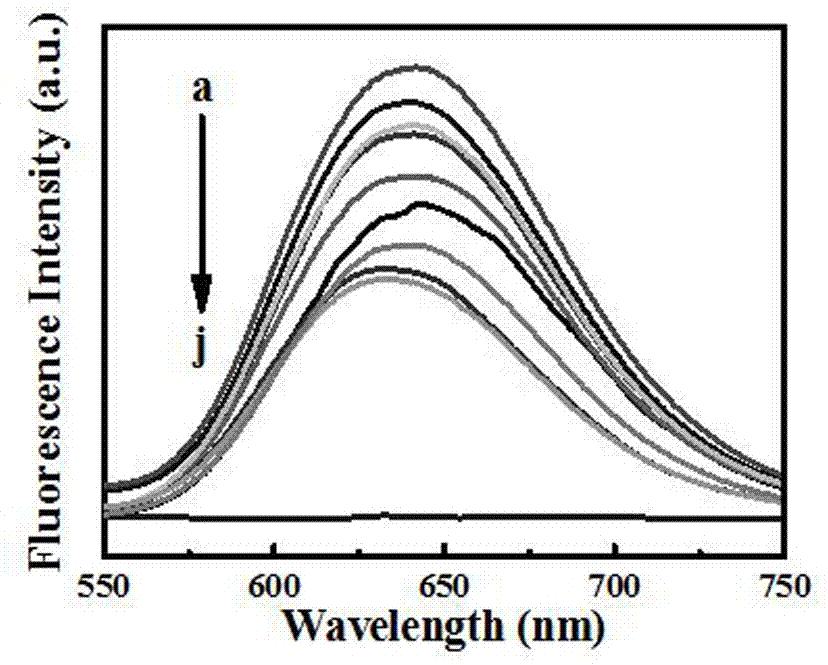
[0045] The alcohol-induced fluorescence-enhanced silver nanocluster probes were sequentially added to an equal volume (100 μl) with a concentration gradient (0.050, 0.10, 0.25, 0.50, 0.75, 1.0, 2.5, 5.0, 7.5, and 10 μM). I - In the urine, the probe was added at a concentration of 1.05 mM, using a 1.0 M HCl or NaOH aqueous solution, adjusting the pH to 6-8, reacting for 5 min, and observing the color of the solution under a UV projector with a wavelength of 365 nm Use a fluorescence spectrometer to measure the fluorescence spectrum and intensity at the maximum excitation wavelength of 425nm, and draw a calibration curve; according to the above method, measure the fluore...
Embodiment 3
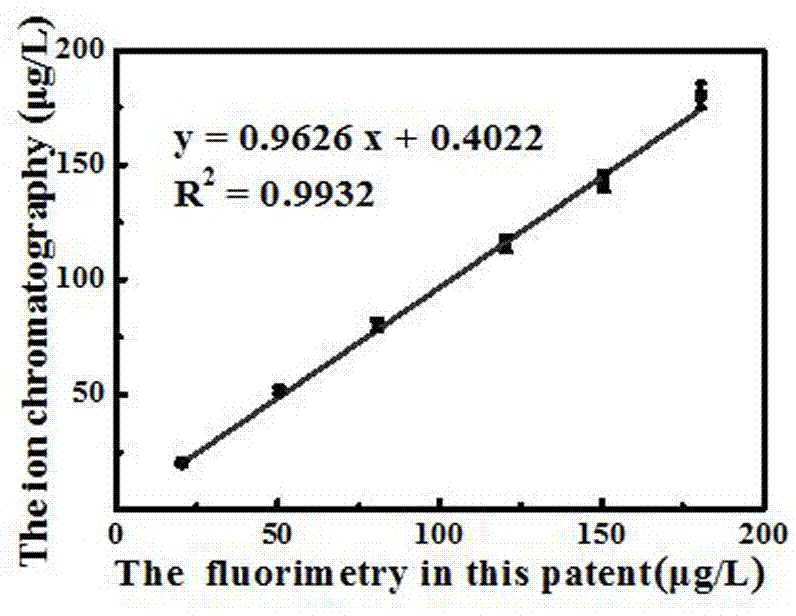
[0047] Urine iodine ion detection method based on alcohol solvent-induced fluorescence enhancement of silver nanoclusters, the alcohol-induced fluorescence-enhanced silver nanocluster probe prepared in Example 1 is used to determine iodine ions in urine to be tested (morning urine of pregnant women), and the steps are the same Example 2, the results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com