A kind of piribedil sustained-release tablet and preparation method thereof
A technology for piribedil and sustained-release tablets, which is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. phenomenon and other problems, to achieve a good slow-release effect, overcome difficulties in regulation, and expand production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
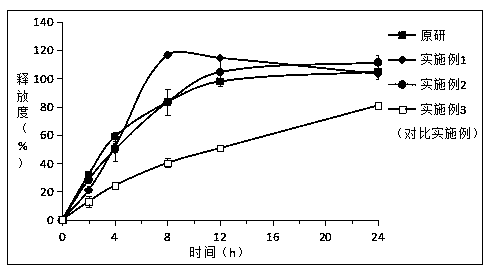
[0032] Embodiment 1: Preparation of piribedil sustained-release tablets
[0033] Take by weighing 2.5g piribedil, 2.5g glyceryl palmitate stearate, 5g microcrystalline cellulose, cross 80 mesh sieves after mixing uniformly according to the equivalent incremental method, the waxy molten liquid of 2.5g (0.5g white beeswax , 1g palm wax and 1g PEG 6000 Add ethanol, melt at 85°C and dry to remove ethanol) Add the main drug, microcrystalline cellulose and glyceryl palmitate stearate into the mixture, use the ethanol solution of PVPK30 (concentration: 5%wt.) as the binder (solution Use amount 10g) to make soft material, granulate with 30 mesh sieve, dry at 60°C for 1 hour, granulate with 30 mesh sieve, add 0.2g magnesium stearate and 0.3g silicon dioxide, mix well, and press into tablets.
Embodiment 2
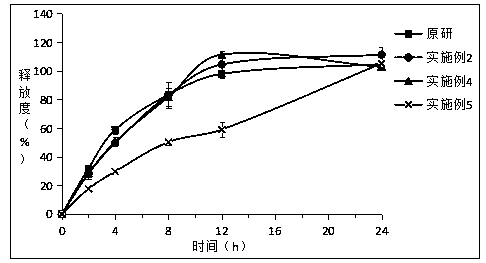
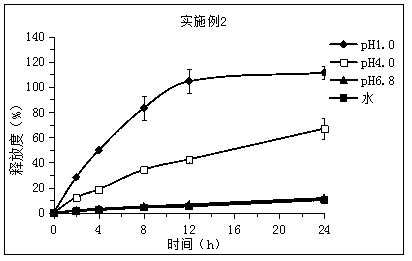
[0034] Embodiment 2: Preparation of piribedil sustained-release tablets
[0035] Take by weighing 2.5g piribedil, 5g glyceryl palmitate stearate, 2.5g microcrystalline cellulose, cross 80 mesh sieves after mixing uniformly according to the equivalent incremental method, the waxy molten liquid of 2.5g (0.5g white beeswax , 1g palm wax and 1g PEG 6000 Add ethanol, melt at 85°C and dry to remove ethanol) Add the main drug, microcrystalline cellulose and glyceryl palmitate stearate into the mixture, use the ethanol solution of PVPK30 (concentration: 5%wt.) as the binder (solution Use amount 10g) to make soft material, granulate with 30 mesh sieve, dry at 60°C for 1 hour, granulate with 30 mesh sieve, add 0.2g magnesium stearate and 0.3g silicon dioxide, mix well, and press into tablets.
Embodiment 3
[0036]Embodiment 3: Preparation of piribedil sustained-release tablets (comparative example)
[0037] Take by weighing 2.5g piribedil, 7.5g glyceryl palmitostearate, cross 80 mesh sieves after mixing uniformly according to the equal amount addition method, the waxy molten liquid of 2.5g (0.5g white beeswax, 1g palm wax and 1gPEG 6000 Add ethanol and melt at 85°C) into the mixture of the main drug, microcrystalline cellulose and glyceryl palmitate stearate, and use the ethanol solution of PVP K30 (concentration: 5%wt.) as the binder (the amount of the solution is 10g) To make soft material, granulate with a 30-mesh sieve, dry at 60°C for 1 hour, granulate with a 30-mesh sieve, add 0.2g magnesium stearate and 0.3g silicon dioxide, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com