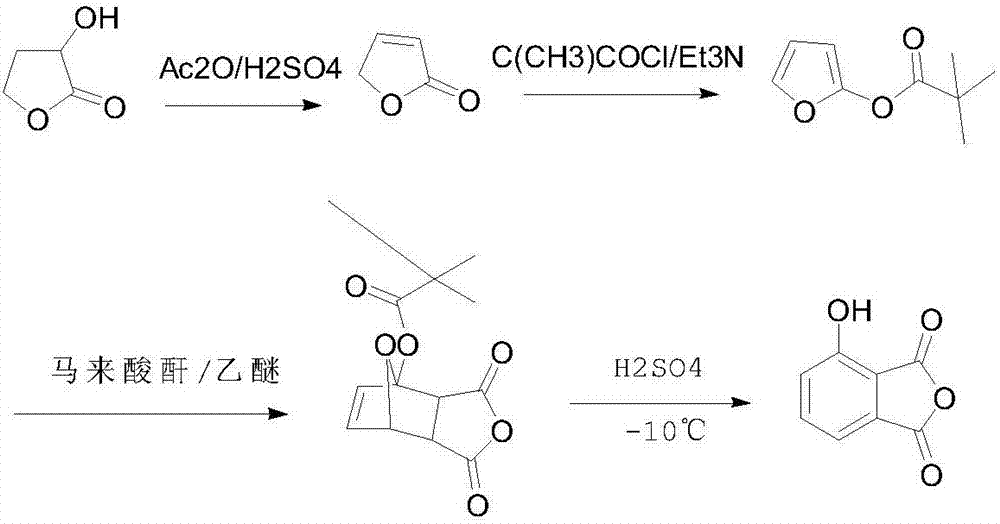
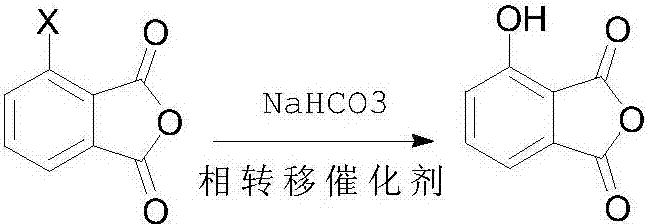
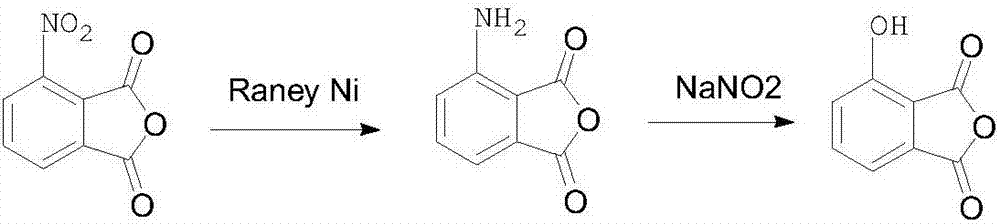
Preparation method of 3-hydroxyl phthalic anhydride
The technology of hydroxyphthalic anhydride and hydroxyphthalic acid is applied in the field of preparation of key pharmaceutical intermediate 3-hydroxyphthalic anhydride, and can solve the problems of complex process steps, high price, low yield and the like, Achieve high yield, good purity and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add DMF100.00g, 1-methoxy-2,3-dimethylbenzene 27.24g (0.20mol) and hexadecyltrimethylammonium bromide 14.58g (0.04mol) to a 500mL three-necked flask, heat up When the temperature reaches 70°C, start to add 79.01g (0.50mol) of potassium permanganate in batches, control the temperature not to exceed 90°C, and finish adding in about 30 minutes. , filtered, washed with 100.00g of water*3 to obtain off-white solid, dried at 55-60°C, 39.23g theoretically, 30.11g actually obtained, yield 76.75%, purity 98.70%.
[0027] Add 160.01 g of dichloromethane and 30.00 g (0.15 mol) of 3-methoxyphthalic acid into a 500 mL three-necked flask, and add 76.67 g (0.31 mol) of boron tribromide dropwise in a water bath with temperature control at 10-15 ° C, dropwise After the addition was completed, the insulation reaction was continued for 6 h. Add 400.00g of ice-water mixture into another 1000mL three-neck flask, slowly pour the above reaction solution into ice water, keep the temperature n...
Embodiment 2
[0030] Add water 240.00g, 1-methoxy-2-ethyl-3-toluene 30.04g (0.20mol) and hexadecyltrimethylammonium bromide 7.29g (0.02mol) in the there-necked flask of 500mL, heat up To 80°C, start to add 63.21g (0.40mol) of potassium permanganate in batches, control the temperature not to exceed 90°C, and complete the addition in about 30 minutes. g*3 washed to obtain an off-white solid, dried at 55-60°C, theoretically 39.23g, actual obtained 32.17g, yield 82.00%, purity 98.32%.
[0031] Add 290.00 g of dichloromethane and 30.00 g (0.15 mol) of 3-methoxyphthalic acid into a 500 mL three-necked flask, and add 51.00 g (0.38 mol) of anhydrous aluminum trichloride in batches in a water bath controlled at 25-30°C. ), after adding, continue the insulation reaction for 7.5h. Add 600.00g of ice-water mixture into another 1000mL three-necked flask, slowly pour the above reaction solution into ice water, keep the temperature not exceeding 30°C, then continue stirring for 1 hour, filter, wash with ...
Embodiment 3
[0034] Add DMSO120.00g, 5-methoxy-1,2,3,4-tetrahydronaphthalene 32.45g (0.20mol) and hexadecyltrimethylammonium bromide 36.44g (0.10mol) into a 1000mL three-necked flask ), heat up to 75°C, start adding 110.62g (0.70mol) of potassium permanganate in batches, control the temperature not to exceed 90°C, finish adding in about 40 minutes, keep warm at 80-90°C for 2.5h after adding, cool down to room temperature, Add 600g of water dropwise, filter, wash with 100.00g of water*3 to obtain an off-white solid, dry at 55-60°C, theoretically 39.23g, actual 28.07g, yield 71.55%, purity 98.41%.
[0035] Add 150.00 g of 48% hydrobromic acid and 25.00 g (0.13 mol) of 3-methoxyphthalic acid into a 500 mL three-necked flask, and stir at 25-30°C for 5 h. Add 500.00g of ice-water mixture into another 1000mL three-neck flask, slowly pour the above reaction solution into ice water, keep the temperature not exceeding 30°C, then continue to stir for 1 hour, filter, wash with water until pH = 6, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com