A method for measuring dalbavancin impurities
A dalbavancin and detection method technology, applied in the field of medical analysis, can solve problems such as poor solubility, ineffective liquid phase extraction, and ineffective free vaporization of 3-dimethylaminopropylamine, and achieve low equipment requirements, improve quality and The effect of drug safety and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Chromatographic conditions:
[0073] Column temperature: programmed temperature rise: the initial temperature was 60°C and kept for 8 minutes, then the temperature was raised to 220°C at a rate of 20°C per minute and kept for 8 minutes.
[0074] Injection port temperature: 250°C;
[0075] Flame ionization detector temperature: 300°C;
[0076] Carrier gas: nitrogen;
[0077] Carrier gas flow rate: 1.5ml / min;
[0078] Split ratio: 5:1.
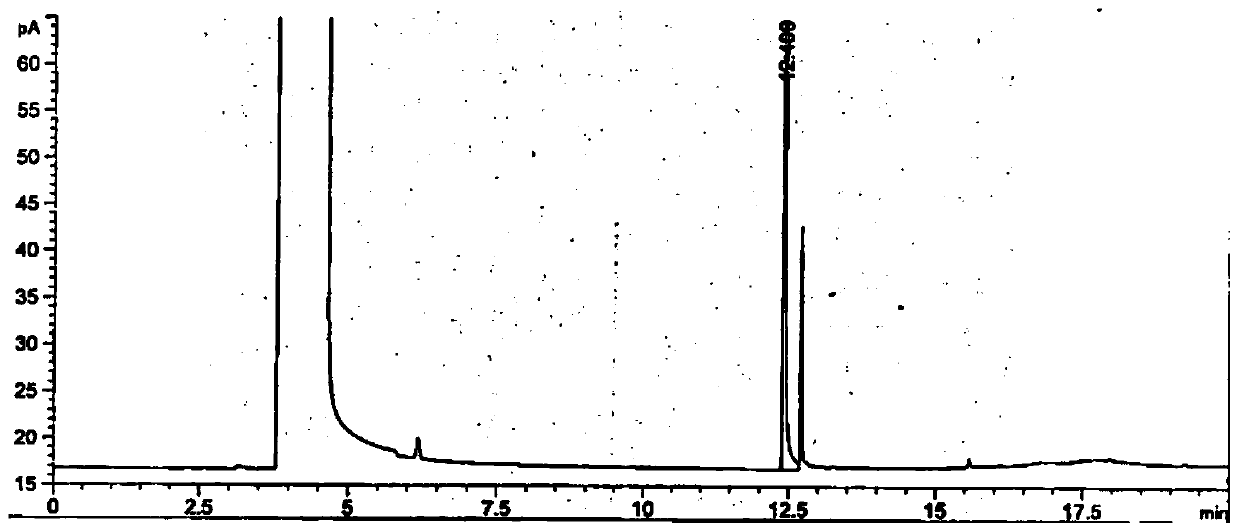
[0079] 1. Detection
[0080] A. Preparation of the test solution:
[0081] Prepare 2.5mol / L sodium hydroxide solution for subsequent use;
[0082] Take about 0.1g of dalbavancin, weigh it accurately, put it in a stoppered test tube, add 1ml of prepared sodium hydroxide solution, shake to dissolve, add 1ml of acetonitrile, add 0.5g of sodium chloride, shake to mix thoroughly, Leave to stand for stratification, absorb the supernatant, and use it as the test solution for subsequent use;
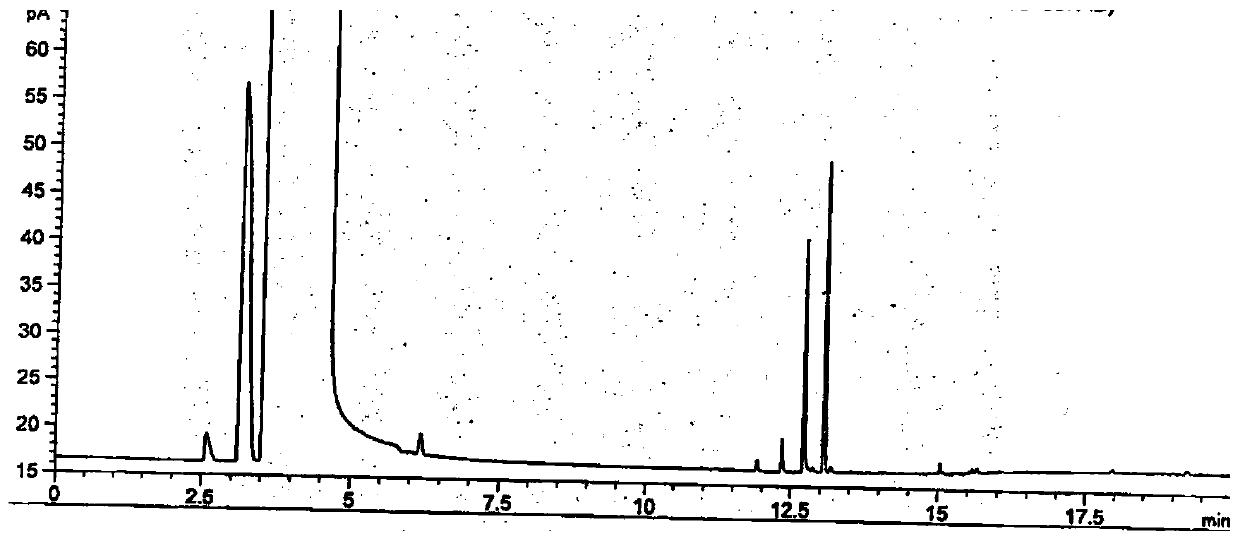
[0083] B. Preparation of reference substance ...
Embodiment 2
[0132] The test sample and reference substance solutions were prepared with reference to Example 1. The difference is that the concentration of the sodium hydroxide solution in this embodiment is 2.0mol / L; the concentration of acetonitrile is 2ml; the inorganic salt is potassium chloride, and the addition amount is 2g.
[0133] Chromatographic conditions:
[0134] Column temperature: programmed temperature rise: the initial temperature was 60°C and kept for 8 minutes, then the temperature was raised to 220°C at a rate of 20°C per minute and kept for 8 minutes.
[0135] Injection port temperature: 230°C;
[0136] Flame ionization detector temperature: 280°C;
[0137] Carrier gas: nitrogen;
[0138] Carrier gas flow rate: 1.0ml / min;
[0139] Split ratio: 1:1;
[0140] The injection volume was 1 μl.
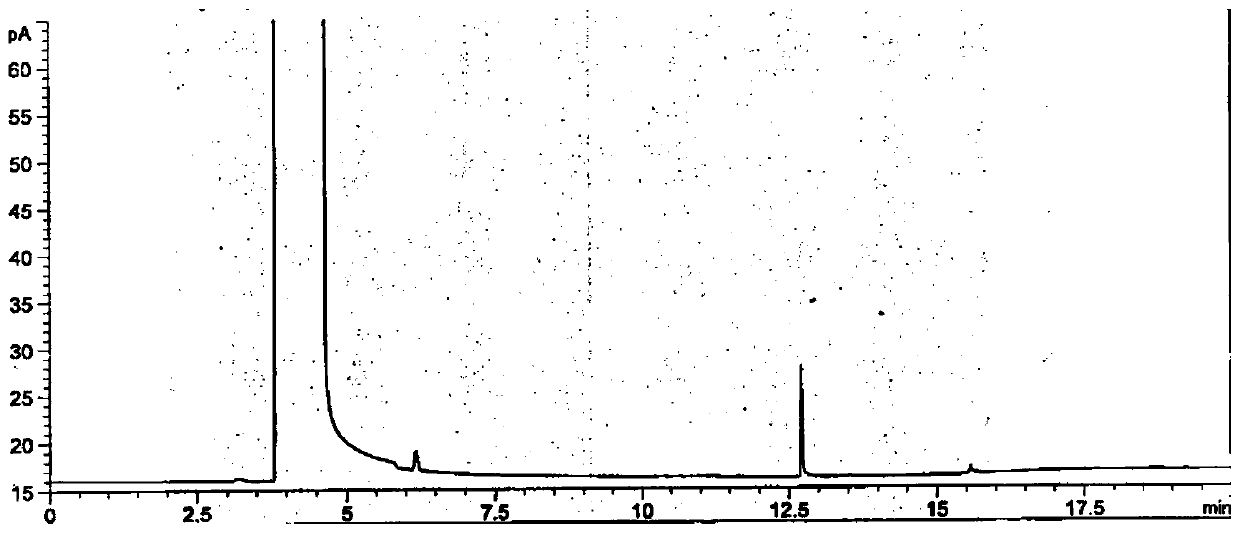
[0141] According to the above conditions, three batches of the test solution were detected, and no 3-dimethylaminopropylamine peak was detected in the chromatograms. After cal...
Embodiment 3
[0143] The test sample and reference substance solutions were prepared with reference to Example 1. The difference is that the sodium hydroxide solution in this embodiment is 10ml, the concentration is 3.0mol / L; the acetonitrile is 1ml; the inorganic salt is anhydrous sodium sulfate, and the addition amount is 1g.
[0144] Chromatographic conditions:
[0145] Column temperature: programmed temperature rise: the initial temperature was 60°C and kept for 8 minutes, then the temperature was raised to 220°C at a rate of 20°C per minute and kept for 8 minutes.
[0146] Injection port temperature: 270°C;
[0147] Flame ionization detector temperature: 320°C;
[0148] Carrier gas: nitrogen;
[0149] Carrier gas flow rate: 2.0ml / min;
[0150] Split ratio: 10:1;
[0151] The injection volume was 3 μl.
[0152] According to the above conditions, three batches of the test solution were detected, and no 3-dimethylaminopropylamine peak was detected in the chromatograms. After calcula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com