HCV (hepatitis C virus) recombinant fusion antigen and application thereof
A fusion antigen and C-terminal technology, applied in the direction of fusion peptide, recombinant DNA technology, hybrid peptide, etc., can solve the problems of difficult adsorption, low specificity, and small molecular weight of synthetic peptides, and achieve the effect of easy renaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Design of HCV recombinant fusion antigen
[0042] Because the antigens of viral infectious diseases are often large, it is difficult to express the complete antigenic protein in vitro, or even if it is expressed in vitro, the antigenic protein with a relatively large molecular weight often cannot be folded correctly, resulting in difficulties in later renaturation, or renaturation. The antigenic activity of the poor, restricting the development of detection reagents. Therefore, using genetic engineering technology, the fusion expression of multiple dominant antigen epitope genes has become the direction of antigen research. Through the screening of a large number of experiments, the present invention finally determines to select two dominant epitopes of core (2-102aa) and NS5 (2322-2422aa), optimize the codons and connect them in series through the flexible link GGGSGG with strong hydrophilicity . The epitope of the selected Core is 2-102aa, its amino acid s...
Embodiment 2
[0048] Example 2 Construction of HCV Recombinant Fusion Antigen Expression Vector
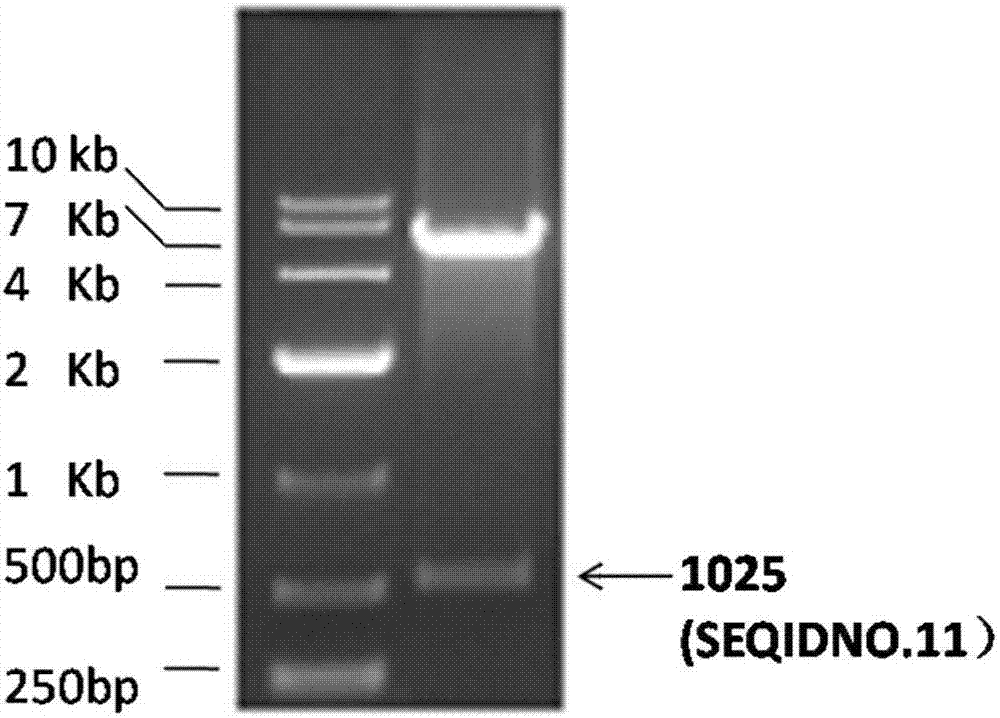
[0049] The target gene fragment 1025 (SEQ ID NO.11) obtained in Example 1 and the expression vector pET28a were subjected to double digestion with restriction endonucleases EcoRI and XhoI, respectively.
[0050] The enzyme digestion reaction system of target gene fragment 1025 is:
[0051]
[0052] The enzyme digestion reaction system of the expression vector pET28a is:
[0053]
[0054] Enzymatic digestion in a water bath at 37°C overnight. The digested products were detected by 1% TAE agarose gel electrophoresis. Recover with Axygen Mini Gel Recovery Kit. Ligated at 16°C for 4 hours, and transferred the ligated product into competent E.coli DH5α cells by heat shock method, and spread it on a medium containing 50 μg / ml Kan + On the LB agar medium, under the condition of 37 ℃, culture upside down overnight or 12-14 hours until a single colony grows.
[0055] Initial screening was car...
Embodiment 3
[0066] Example 3 Induced Expression of HCV Recombinant Fusion Antigen
[0067] Transform Escherichia coli E.coliBL21 (Rossetta) competent cells with the positive recombinant plasmid pET28a-1025 that has been sequenced and identified by restriction enzyme digestion by heat shock method, and spread it on a medium containing 50 μg / ml Kan + and 25 μg / ml Chl + On the LB agar medium, under the condition of 37 ℃, culture upside down overnight or 12-14 hours until a single colony grows.
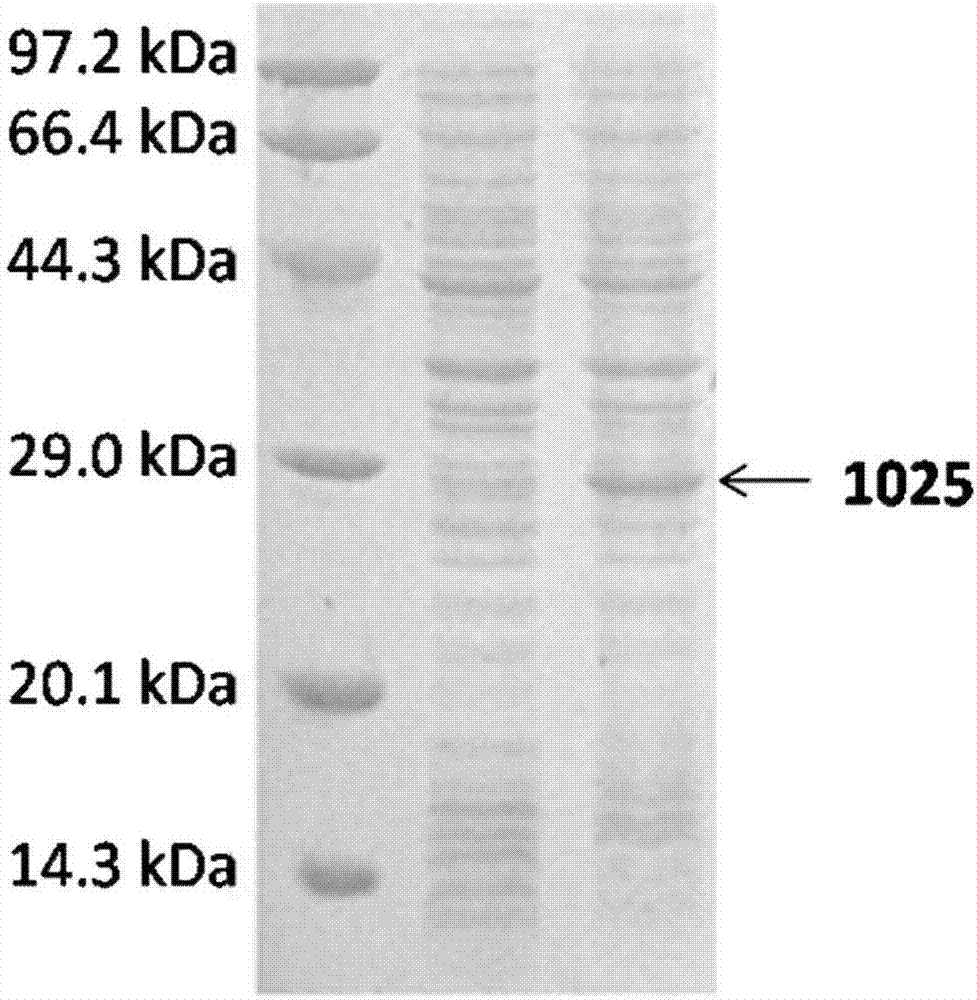
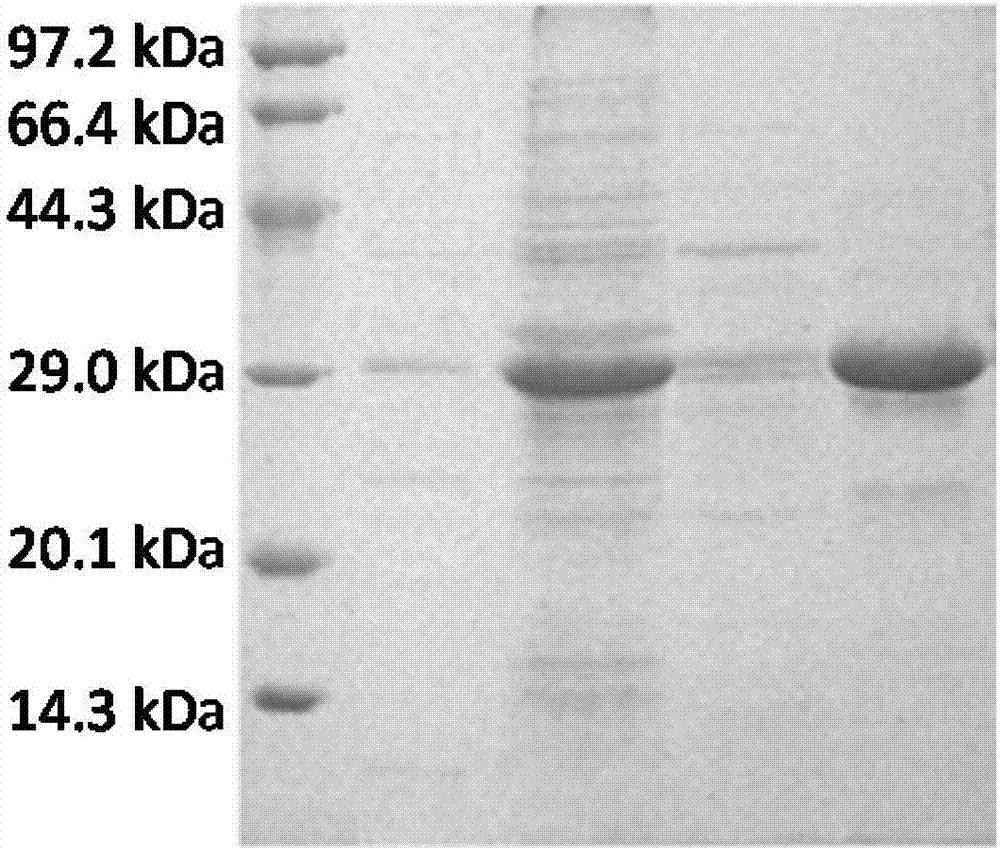
[0068] Pick 2 single colonies and inoculate to contain 50μg / ml Kan + and 25 μg / ml Chl + In 5ml LB liquid medium, culture overnight at 37°C, 200r / min. On the next day, transfer 200 μl of bacterial solution to 5 ml of fresh 50 μg / ml Kan + and 25 μg / ml Chl + LB liquid medium, 37°C, 200r / min culture to OD600≈0.8. Add IPTG to a final concentration of 1 mM, and continue shaking culture at 37° C. for 4 hours. SDS-PAGE electrophoresis was performed after induction (see figure 2 ). The cloned bacter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com