Method for asymmetrically opening ring of 3-substituted oxetane and application
An oxetane, asymmetric technology, applied in the field of asymmetric catalytic synthesis, can solve the problems of catalyst deactivation, weak ring-opening reaction activity, and high difficulty in selecting nucleophiles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
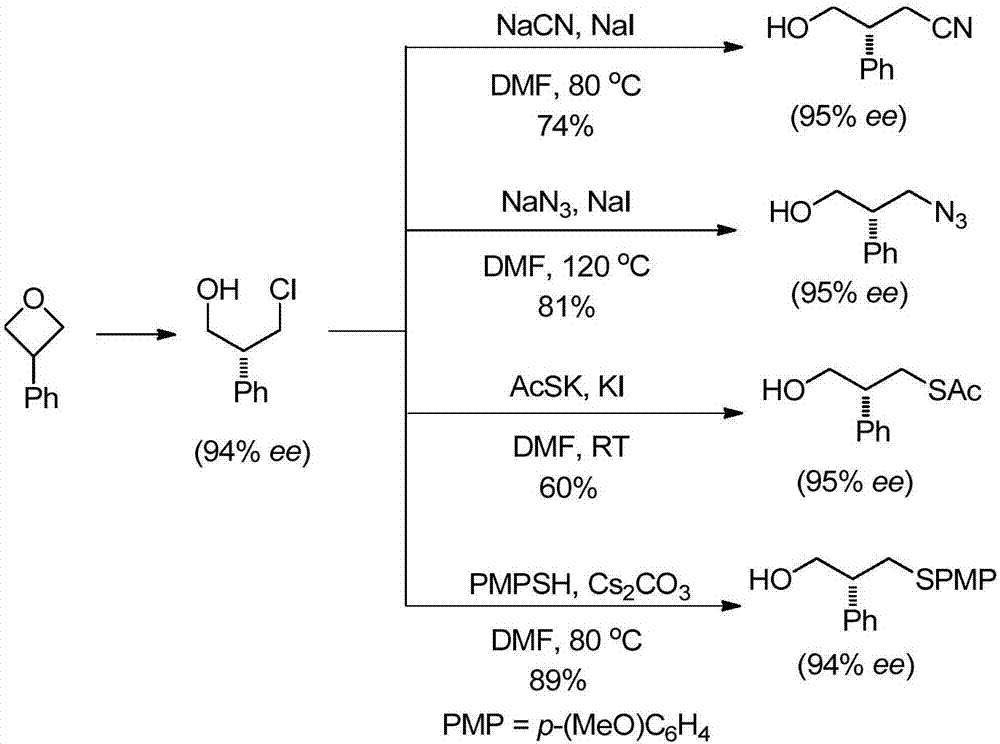
Image
Examples
Embodiment 1
[0032] Step Step 1, preparing the precursor compound S3 of the chiral phosphoric acid catalyst CAT:
[0033] (1) Prepare Grignard reagent S2. Under nitrogen protection, magnesium powder (1.44 g, 60.0 mmol) and anhydrous tetrahydrofuran (15 mL) were placed in a 250 mL round bottom flask, and a small amount of iodine and 1,2-dibromoethane were added as activators. Stir for 20 minutes, then slowly add a solution of 1-bromo-2,4,6-tricyclohexylbenzene (12.10g, 30.0mmol) in tetrahydrofuran (85mL) dropwise to the reaction flask, and the reaction solution is heated to reflux for 12 hours, then cooled Bring to room temperature for later use.
[0034] (2) Precursor compound S3 of chiral phosphoric acid catalyst CAT was prepared by compound S1 and Grignard reagent S2. Under nitrogen protection, compound S1 (2.37 g, 4.0 mmol) and bis(triphenylphosphine)nickel chloride (523 mg, 0.8 mmol) were placed in a 100 mL dry round bottom flask, and anhydrous tetrahydrofuran (20 mL) was added. Und...
Embodiment 2
[0070] In this embodiment, 3-(4-methylphenyl)oxetane is used as the substrate, and the above-mentioned steps are the same as in Example 1, and the catalyst dosage is 2mol%, and the reaction is carried out for 18 hours to obtain the corresponding The reaction product of Example 2, 50.2 mg of colorless oily substance, calculated yield 91%.
[0071] Similarly, after the preparation of this step is completed, the obtained oily product is analyzed by means of measuring specific rotation, high performance liquid chromatography to measure ee value, nuclear magnetic resonance, infrared and high-resolution mass spectrometry. Among them, the analysis of the test is as follows:
[0072] 1. Specific rotation [α] measured by D line at 25°C D 25 :-24.1 (c=1.0, CHCl 3 );
[0073]2. HPLC analysis and determination of ee value: chiral column Daicel Chiralcel OD-H column; 5% i-PrOH in hexanes; 1.0mL / min; retention time: 10.9min (major), 11.8min (minor). Calculation The result was 94% ee. ...
Embodiment 3
[0082] In this Example 3, 3-(2-methoxyphenyl)oxetane was used as the substrate, followed the same procedure as above, with a catalyst dosage of 5 mol%, and reacted for 24 hours to obtain the corresponding reaction product, colorless 51.2mg of oil, yield 85%.
[0083] Similarly, after the preparation of this step is completed, the obtained oily product is analyzed by means of measuring specific rotation, high performance liquid chromatography to measure ee value, nuclear magnetic resonance, infrared and high-resolution mass spectrometry. Among them, the analysis of the test is as follows:
[0084] 1. Specific rotation measured by D line at 25°C [α] D 25 : -11.4 (c=1.0, CHCl 3 ).
[0085] 2. HPLC analysis and determination of ee value: Chiral column Daicel Chiralcel OD-H column; 5% i-PrOH in hexanes; 1.0mL / min; retention time: 17.4min (major), 13.7min (minor), calculated The result is 90% ee.
[0086] 3. Proton spectrum and carbon spectrum of nuclear magnetic resonance ana...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com