Mithramycin nano-particle suspension and preparation method thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
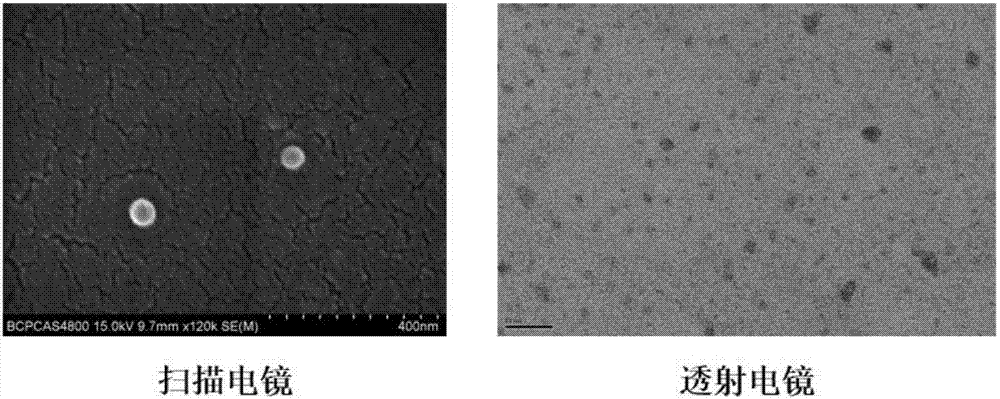
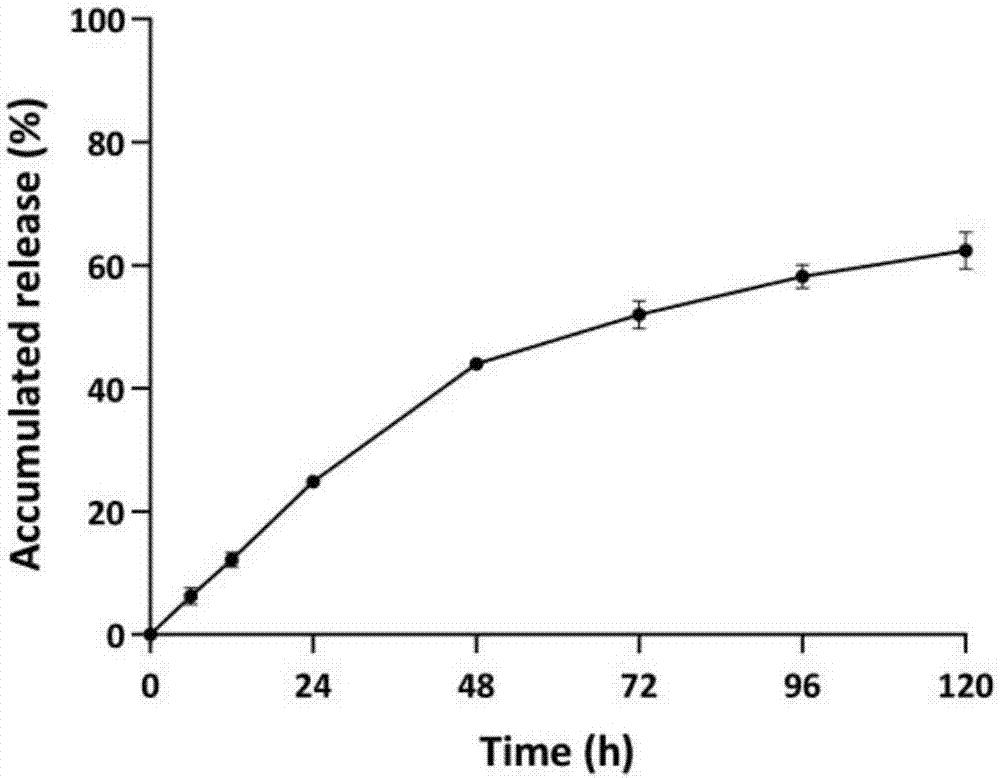
Image
Examples
Embodiment 1
[0108] Example 1: Preparation of a suspension of scintillation nanoparticles
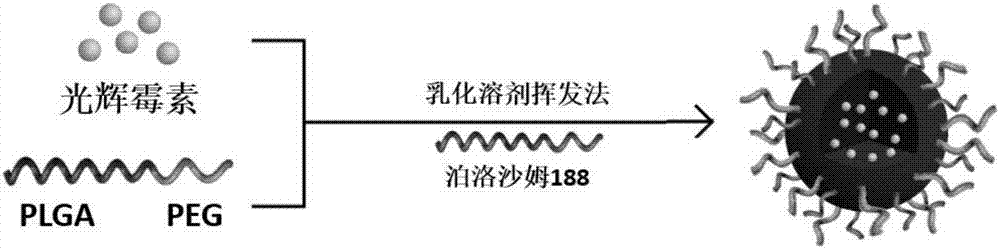
[0109] The basic process of preparing scintilaginous nanoparticles by emulsification solvent evaporation method is shown in the appendix. figure 1 .
[0110] The implementation steps of this embodiment are as follows:
[0111] A. Preparation of diblock copolymer solution
[0112] The mPEG-PLGA diblock copolymer (PEG molecular weight 5000Da; PLGA molecular weight 15000Da; lactic acid and glycolic acid molar ratio: 50 / 50) sold by Jinan Daigang Bioengineering Co., Ltd. was dissolved in ethyl acetate solvent to obtain a concentration of 30 mg / mL diblock copolymer ethyl acetate solution;
[0113] B, the preparation of the organic phase of fucoidin
[0114] To the ethyl acetate solution of the diblock copolymer described above was added the diblock copolymer in the ratio 1:2.2 of the ethyl acetate solution in milliliters to the mithramycin in milligrams, marketed by Sigma-Aldrich under the trade nam...
Embodiment 2
[0123] Example 2: Preparation of a Pseudomycin Nanoparticle Suspension
[0124] The implementation steps of this embodiment are as follows:
[0125] A. Preparation of diblock copolymer solution
[0126] The PEG-PLLA diblock copolymer (PEG molecular weight 3000Da; PLLA molecular weight 15000Da) sold by Jinan Daigang Bioengineering Co., Ltd. was dissolved in dichloromethane solvent to obtain a diblock copolymer dichloromethane with a concentration of 40 mg / mL. solution;
[0127] B, the preparation of the organic phase of fucoidin
[0128] In accordance with the ratio of dichloromethane solution in milliliters to 1:1.0 in milligrams of scintilbomycin SK, the scintilbomycin SK reported by the document JAmChem Soc. 2003 May 14; 125(19): 5745-53 was added to the above Diblock copolymer in dichloromethane solution, shake and dissolve, obtain a kind of organic phase that contains brightmycin SK;
[0129] C. Preparation of emulsifier solution
[0130] The emulsifier PVA is dissolv...
Embodiment 3
[0137] Example 3: Preparation of a Pseudomycin Nanoparticle Suspension
[0138] The implementation steps of this embodiment are as follows:
[0139] A. Preparation of diblock copolymer solution
[0140] The PEG-PDLA diblock copolymer (PEG molecular weight 5000Da; PDLA molecular weight 20000Da) sold by Jinan Daigang Bioengineering Co., Ltd. was dissolved in chloroform solvent to obtain a diblock copolymer chloroform with a concentration of 34mg / mL. solution;
[0141] B, the preparation of the organic phase of fucoidin
[0142] According to the ratio 1:1.6 of chloroform solution in milliliters to the Spotomycin SDK in mg, the Spotsomycin SDK reported by the document JAmChem Soc. 2003 May 14; 125(19): 5745-53 was added to the above-mentioned dimeric In the chloroform solution of the segmented copolymer, vibrating and dissolving to obtain an organic phase containing the Spotomycin SDK;
[0143] C. Preparation of emulsifier solution
[0144] Dissolving the emulsifier sodium ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface charge | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com