Dasatinib nanopreparation and preparation method thereof
A dasatinib and nano-preparation technology, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of long preparation process, complicated preparation process, uneven distribution, etc. , to achieve the effects that are beneficial to the efficacy of the drug, increase the concentration of the drug, and the production process is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
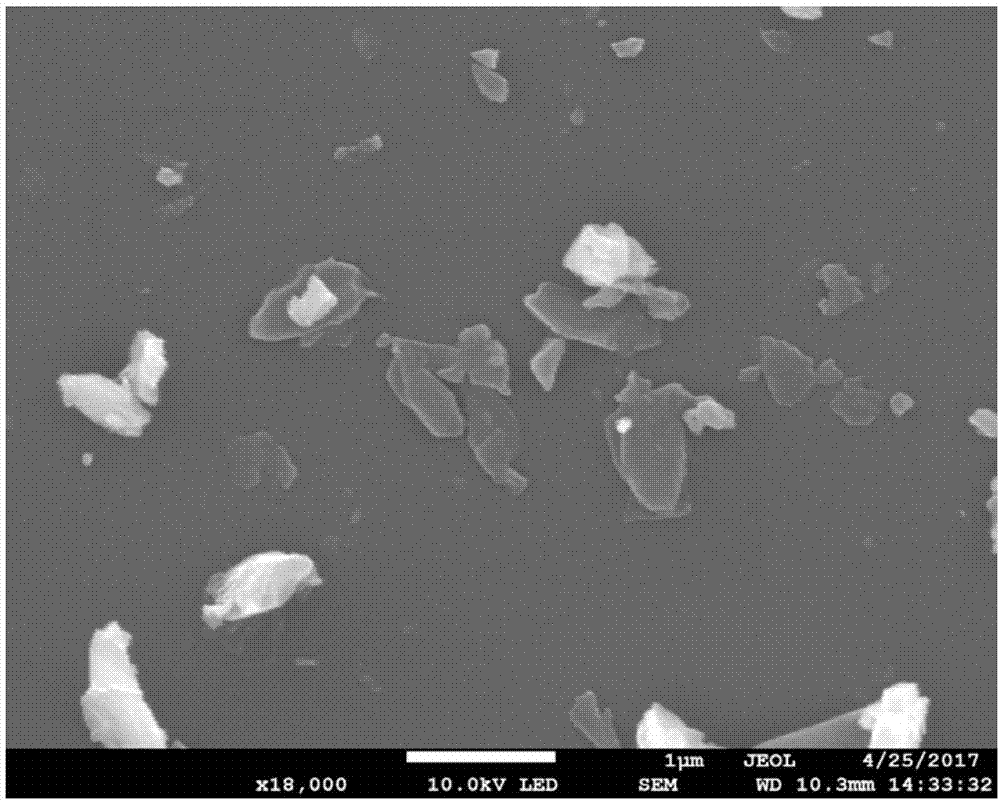
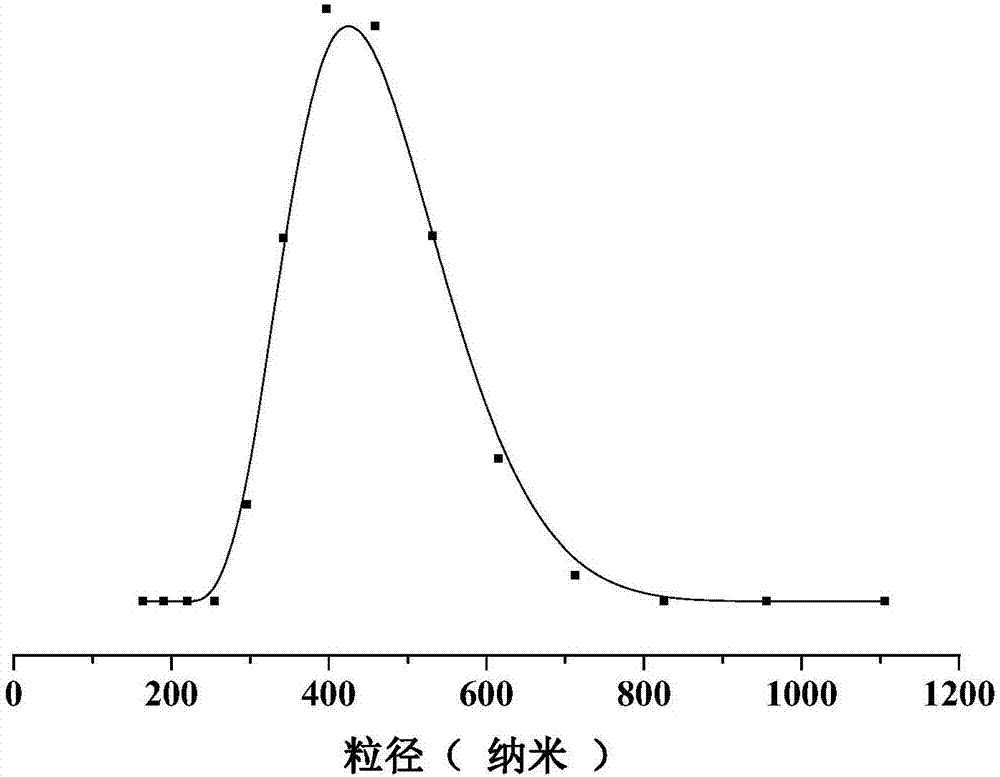
Image
Examples
Embodiment 1
[0049] A dasatinib oral nano-preparation, comprising the following components:
[0050] Dasatinib 600mg, excipients 800mg, additives 600mg;
[0051] Among them, the auxiliary materials include 600 mg of microcrystalline cellulose and 200 mg of crospovidone; the additive is mannitol.
[0052] A preparation method of dasatinib oral nano-preparation, the steps are as follows:
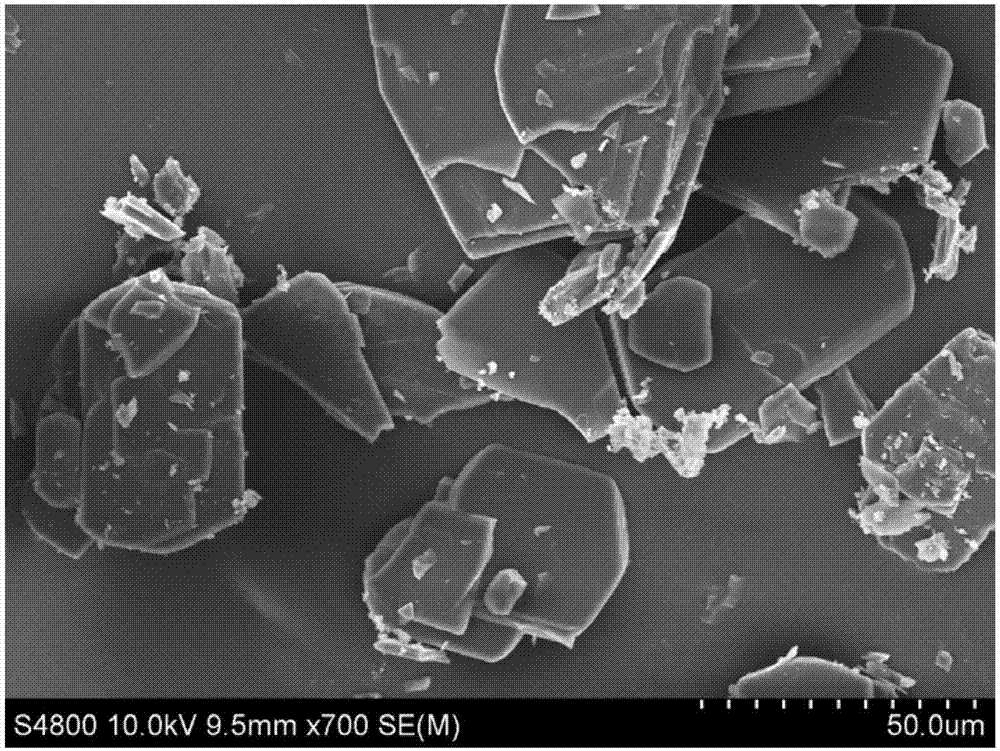
[0053] S1, Dasatinib bulk drug (scanning electron microscope picture as shown in figure 1 shown) and N,N-dimethylformamide to form 20mL of Dasatinib N,N-dimethylformamide solution with a concentration of 30mg / mL;
[0054] S2. Mix mannitol and deionized water into 400 mL of 1.5 mg / mL mannitol aqueous solution, and simultaneously add 15 mg of sodium lauryl sulfate to the mannitol aqueous solution to obtain a mixed solution;
[0055] S3. Add the dasatinib N,N-dimethylformamide solution obtained in step S1 to the mixed solution obtained in step S2, stir with a magnetic stirrer for 35 minutes, and control th...
Embodiment 2
[0065] A dasatinib oral nano-preparation, comprising the following components:
[0066] Dasatinib 800mg, excipients 1100mg, additives 800mg;
[0067] Among them, the auxiliary materials include 1000 mg of polyvinylpyrrolidone and 100 mg of hypromellose; the additive is lactose monohydrate.
[0068] A preparation method of dasatinib oral nano-preparation, the steps are as follows:
[0069] S1, Dasatinib bulk drug and dimethyl sulfoxide are made into 20 mL of dasatinib dimethyl sulfoxide solution with a concentration of 40 mg / mL;
[0070] S2, polyvinylpyrrolidone and deionized water are made into 500mL of polyvinylpyrrolidone aqueous solution of 2mg / mL;
[0071] S3. Open the internal circulation high-gravity rotating packed bed, add the dasatinib dimethyl sulfoxide solution obtained in step S1 and the aqueous solution of pharmaceutical excipients obtained in step S2 into the high-gravity rotating packed bed at the same time, adjust the speed to 2800rpm, and circulate The time...
Embodiment 3
[0077] A dasatinib oral nano-preparation, comprising the following components:
[0078] Dasatinib 500mg, excipients 510mg, additives 300mg;
[0079] Wherein, the auxiliary materials include 10 mg sodium dodecyl sulfonate, 400 mg microcrystalline cellulose, 50 mg carboxymethyl cellulose sodium and 50 mg hypromellose; the additive is mannitol.
[0080] A preparation method of dasatinib oral nano-preparation, the steps are as follows:
[0081] S1. Dasatinib bulk drug and N, N-dimethylacetamide were formulated into 10 mL of Dasatinib N, N-dimethylacetamide solution with a concentration of 50 mg / mL;
[0082] S2. Mannitol and deionized water are mixed into 200 mL of 1.5 mg / mL mannitol aqueous solution, and 10 mg of sodium dodecylsulfonate is added to the mannitol aqueous solution at the same time to obtain a mixed solution
[0083] S3. Add the dasatinib N,N-dimethylacetamide solution obtained in step S1 to the mixed solution obtained in step S2, stir for 15 minutes using a high-sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com