Conversion method for euphorbia lathyris phorbol derivative and use thereof for preparing antitumor drug
A technology of escapillium and diterpene alcohol, which is applied in the field of biological medicine and can solve the problems of microbial transformation research and difficult-to-transform products of diterpenoid compounds that have not yet been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
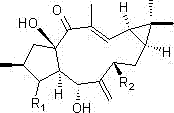
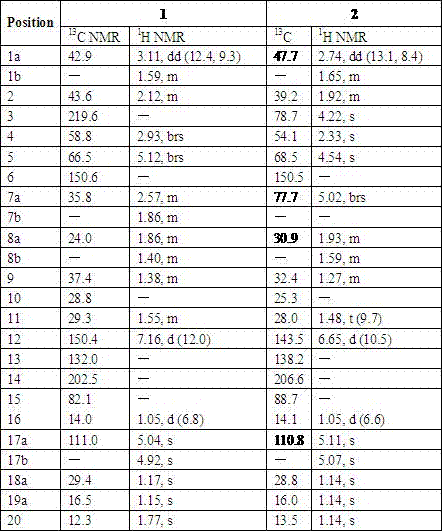
[0023] Example 1 Preparation of 3-carbonyl Capper diterpene alcohol (1)
[0024] The two-step activation method was used to activate the strains, and the obtained seed liquid was inoculated into 250 mL potato medium with a volume of 1 L Erlenmeyer flask at a volume ratio of 3%, and the shake flask was cultured at 28 °C and 180 rpm for 72 h. Add 4 mg / mL Capper diterpene alcohol ethanol solution to each bottle of activated bacterial liquid, and the final concentration is 0.1 mg / mL. After culturing under the same conditions for 72 h, 1.5 times the volume of ethyl acetate was added to extract three times (about 1200 mL), and the organic layers were combined. The organic layer with residual bacteria was suction-filtered through a Buchner funnel to obtain a clarified extract, and then the ethyl acetate was recovered under reduced pressure to obtain a fermentation broth extract;
[0025] The extract of the fermentation broth was dissolved in a small amount of ethyl acetate, mixed wi...
Embodiment 2
[0026] Example 2 Preparation of 7β-acetoxy Capper diterpene alcohol (2)
[0027] The two-step activation method was used to activate the strains, and the obtained seed liquid was inoculated into 250 mL potato medium with a volume of 1 L Erlenmeyer flask at a volume ratio of 3%, and the shake flask was cultured at 28 °C and 180 rpm for 72 h. Add 5 mg / mL ethanol solution of 7β-hydroxycapricorn diterpene alcohol to each bottle of activated bacterial liquid, and the final concentration is 0.1 mg / mL. After culturing under the same conditions for 72 h, 1.5 times the volume of ethyl acetate was added to extract three times (about 1200 mL), and the organic layers were combined. The organic layer with residual bacteria was pumped through a Buchner funnel to obtain a clarified extract, and then the ethyl acetate was recovered under reduced pressure to obtain a fermentation broth extract;
[0028] The extract of the fermentation broth was dissolved in a small amount of ethyl acetate, mi...
Embodiment 3
[0032] Example 3 Antitumor Activity Experiment of 3-Carbonyl Scaper Diterpene Alcohol (1)
[0033]Human breast cancer cells (MCF-7) and human colon cancer cells (Caco-2) were used as models. MCF-7 cell culture medium is RPMI-1640 medium containing 10% calf serum, and Caco-2 cell culture medium is DMEM medium containing 10% calf serum and 1% glutamic acid; cells are treated with 10% trypsin After digestion, the medium was blown to make a single cell suspension, and after counting, the cell concentration was adjusted to 10 5 cells / mL, the cells were seeded in a 96-well plate with a volume of 100 mL per well. After 37℃, 5% CO 2 After incubation for 24 h, the supernatant was discarded, and 200 mL of drug-containing medium was added to each group of cells, and 3 replicate wells were set up for each concentration; the experimental group was added with different concentrations of compound 1 and DMSO solution of Capper diterpene alcohol (100.0 , 80.0, 50.0, 40.0, 25.0, 20.0, 12.5, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com