Preparation method of Zn/adhesive-free ZSM-11 molecular sieve catalyst
A ZSM-11, molecular sieve technology, applied in molecular sieve catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., to achieve the effect of high conversion rate of butane and yield of aromatics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
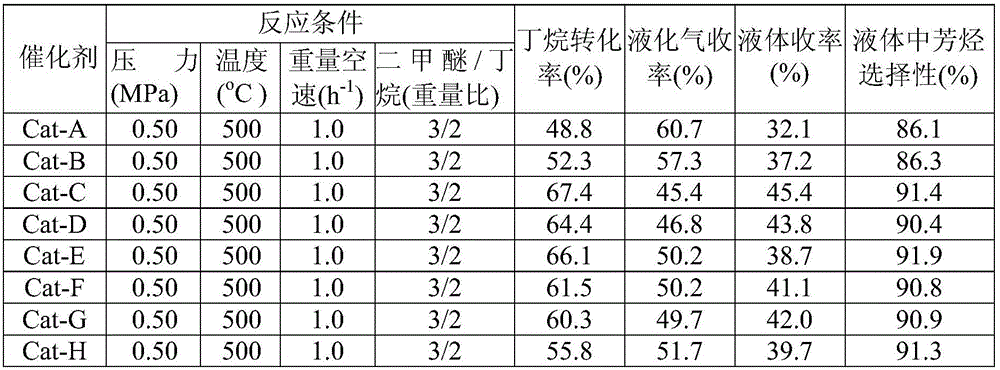
[0031] Sample-B was prepared according to the method of Comparative Example 2, and then metal zinc was loaded by hydrothermal deposition. The loading amount of zinc was 4.0wt%. Specifically, 10 grams of Sample-B was dipped in 30ml of zinc nitrate (0.21mol / L) and In the mixed solution of urea (zinc nitrate / urea=3 / 1 weight ratio), the hydrothermal temperature in the autoclave is 120℃, and the reaction is 20h. The precipitated mixture is filtered, and then placed in a drying oven at 120℃. 3h, then calcined at 600℃ for 4h to prepare Cat-C. The obtained catalyst Cat-C was tested by XRF, and the Na in the catalyst 2 O is less than 0.05wt%.
Embodiment 2
[0033] Weigh 10 grams of ZSM-11 molecular sieve, add 12 grams of 40% (weight) silica sol to mix, extrude and shape, and dry at 120°C to obtain sample B; add 10 grams of 1,6-hexanedi to the reactor in advance A mixture of amine and 20 grams of distilled water, 10 grams of sample B was placed on a porous stainless steel mesh in the reactor and sealed, and then subjected to gas-solid phase treatment at 180°C for 24 hours. The product was taken out and washed with distilled water, dried, roasted at 550°C for 2h, exchanged with 0.6mol / L ammonium nitrate solution at 80°C for 3 times, washed twice with water, dried at 120°C, roasted at 570°C for 4h, then steamed at 625°C After 3 hours of treatment, the obtained catalyst was recorded as Sample-C. XRD results show that the relative crystallinity of Sample-C is 145%.
[0034] Sample-C is loaded with zinc metal by hydrothermal deposition method, the loading amount of zinc is 6.0wt%, specifically, 10g of Sample-C is immersed in 30ml of a mi...
Embodiment 3
[0036] Weigh 10 grams of ZSM-11 molecular sieve, add 40 grams of water glass with a silica content of 20% by weight, mix, extrude, and dry at 120°C to obtain sample C. A mixture of 4 grams of 1,6-hexamethylene diamine and 15 grams of distilled water was added to the reactor in advance, and 10 grams of sample C was placed on the porous stainless steel mesh in the reactor and sealed, and then subjected to gas-solid phase treatment at 120° C. for 90 hours. The product was taken out and washed with distilled water, dried, roasted at 550℃ for 2h, exchanged twice with 0.7mol / L ammonium nitrate solution at 85℃, washed twice with water, dried at 140℃, roasted at 550℃ for 3h, then steamed at 400℃ After 5 hours of treatment, the obtained catalyst was recorded as Sample-D. XRD results show that the relative crystallinity of Sample-D is 142%.
[0037] Sample-D is loaded with zinc metal by hydrothermal deposition method. The loading amount of zinc is 7.0wt%. Specifically, 10g of Sample-D is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallinity | aaaaa | aaaaa |
| crystallinity | aaaaa | aaaaa |
| crystallinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com