Phaseolus vulgaris epoxide hydrolase and heterologous expression method thereof
A technology of epoxide and hydrolase, which is applied in the field of bioengineering, can solve the problems of waste of vicinal diols, inapplicability, and unsatisfactory properties of EHs, and achieve the effect of improving the purity of enantiomers and avoiding low eep
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The primer design of embodiment 1pveh4
[0031] A pair of specific primers were designed according to the primary nucleotide sequence of pveh4 for cloning pveh4. The primer sequences are as follows:
[0032] pveh4-F: GAATTC ATGGAGAACATACTTCACAGAAT (EcoR Ⅰ)
[0033] pveh4-R: CTCGAG TCAGAACTGCTTAATGAAGTCATAAATGT(Xho Ⅰ)
Embodiment 2
[0034] Gene cloning of embodiment 2pveh4
[0035]Choose kidney beans with full grains, soak them at 30°C for 8 hours, and incubate them for 20 hours. Take 100mg germ to extract total RNA. Using total bean RNA as a template and Oligo dT-Adaptor as a primer, the first strand of cDNA was synthesized by reverse transcription; using this strand as a template, PvEH4-F and M13 Primer M4 as primers, the first round of PCR was performed: denaturation at 94°C for 2 min, 30 cycle (94°C for 30s, 50°C for 30s, and 72°C for 70s); then use the first-round PCR product as a template and pveh4-F and pveh4-R as primers for the second round of PCR: denaturation at 94°C for 2 min, 30 cycles (94°C for 30s, 52°C for 30s and 72°C for 70s), 72°C for 10min. The PCR product was analyzed by agarose gel electrophoresis, and the results showed that there was a target band at about 1000bp that was in line with the expected size. The target band was recovered by tapping the gel and connected to pUCm-T, tra...
Embodiment 3
[0036] The heterologous expression of embodiment 3PvEH4
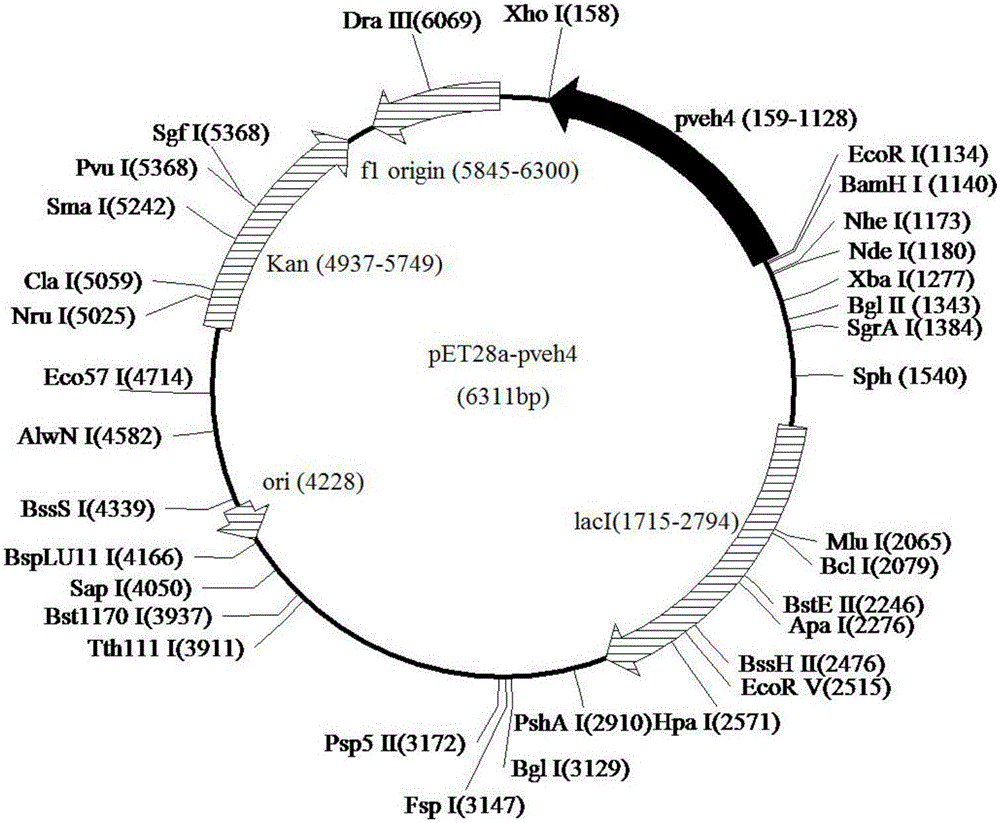
[0037] The pUCm-T-Pveh4 and pET-28a(+) plasmids were simultaneously double-digested with EcoR Ⅰ and Xho Ⅰ, analyzed by agarose gel electrophoresis, and the Pveh4 and pET-28a(+) fragments were recovered by tapping the gel, and T4 DNA ligase was used to Connect overnight to obtain the recombinant plasmid pET28a-pveh4, transform it into E.coli BL21(DE3) competent, pick a single colony and culture it in 2 mL of LB containing 100 μg / mL kanamycin for 4 hours, then carry out bacterial liquid PCR verification, Correct transformants will be verified for DNA sequencing. The correct transformant was named E.coli / pET28a-pveh4.
[0038] Pick a single colony of E.coli / pET28a-pveh4 and E.coli / pET28a and inoculate it in 2 mL of LB medium containing 100 μg / mL kanamycin, and cultivate overnight at 37°C and 220 r / min; take 2 mL of the culture medium to transfer In 100mL of the same medium, cultivate to OD 600 When it is 0.6-0.8, add IP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com