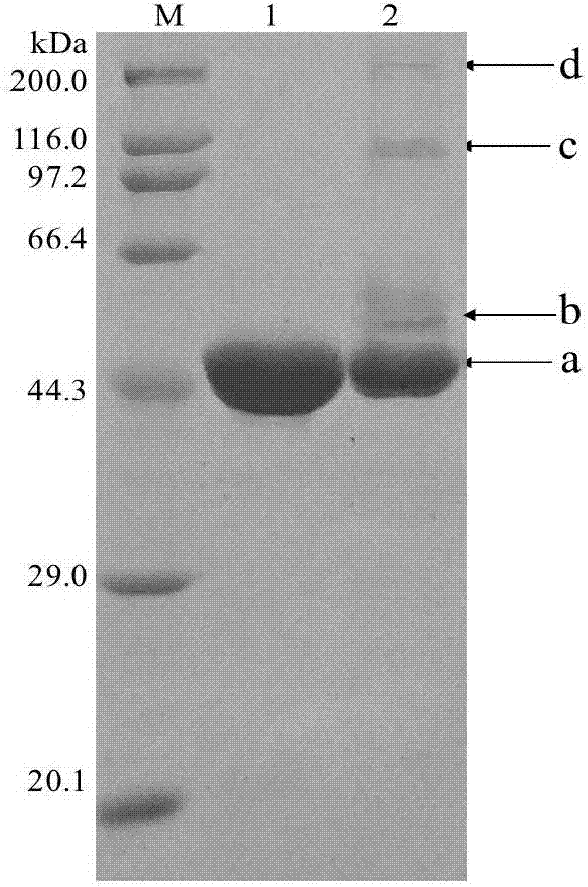
Method for improving pH stability of creatinase
A technology of creatinase and stability, applied in the field of enzyme engineering, can solve the problems such as rarely seen creatinase, and achieve the effect of being suitable for industrial application, reducing enzyme inactivation, and convenient application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Analysis of acidic amino acids on the molecular surface of creatinase
[0024] The covalent modification of polylysine depends on the number of acidic amino acids on the surface of the enzyme. According to the analysis of the amino acid sequence and structure of creatinase (PDB ID: 1CHM), creatinase derived from Pseudomonasputida contains 108 acidic amino acids, of which 76 An acidic amino acid is located on the surface of the enzyme molecule, and the side chain functional group is exposed on the surface of the enzyme, which can participate in the modification of the poly-lysine on the surface of the enzyme molecule.
[0025] Embodiment: 2: Polylysine modified creatinase
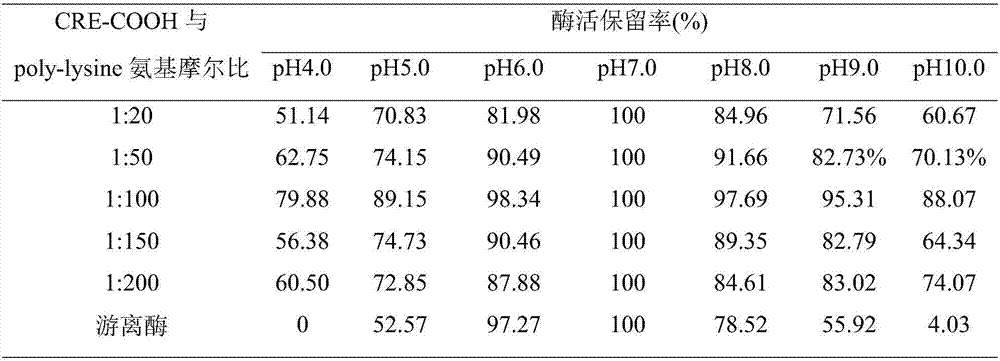
[0026] The molar ratios of carboxyl groups and poly-lysine amino groups on the surface of creatinase were 1:20, 1:50, 1:100, 1:150 and 1:200, respectively.
[0027] The molar ratio of CRE-COOH to EDC was 1:10, the reaction pH was 7.0, and the mixture was mixed slowly at 4°C overnight, and ...
Embodiment 3
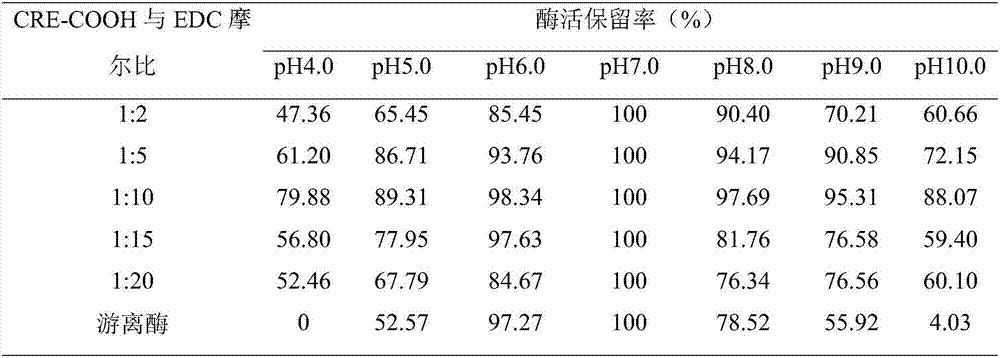
[0030] Embodiment 3: Polylysine modified creatinase
[0031] The molar ratios of carboxyl groups and poly-lysine amino groups on the surface of creatinase were 1:100, the molar ratios of CRE-COOH and EDC were 1:2, 1:5, 1:10, 1:15 and 1:20, and the reaction pH was 7.0, slowly rotate and mix overnight at 4°C, and calculate the relative enzyme activity; ultrafilter the modified enzymes obtained in different molar ratios to remove unbound small molecule modifiers, and place them in buffer solutions with different pH values (pH 4.0, 5.0, 6.0 , 7.0, 8.0, 9.0 and 10.0), placed at 25°C for 16 hours, measured the enzyme activity, calculated the relative activity with the enzyme activity under the optimum pH condition of the free enzyme or modified enzyme as 100%, and determined the pH stability of the enzyme. The results are shown in Table 2.
[0032] Table 2 The pH stability of enzymes modified by the ratio of carboxyl groups and EDC on the surface of different creatinases
[0033...
Embodiment 4
[0034] Embodiment 4: Polylysine modified creatinase
[0035] The molar ratio of carboxyl groups and poly-lysine amino groups on the surface of creatinase is 1:100, the molar ratio of CRE-COOH and EDC is 1:10, the reaction pH is 5.5, 6.5, 7.0, 7.5 and 8.5, and the mixture is mixed slowly at 4°C Overnight, the relative enzyme activity was calculated; the modified enzymes obtained at different pHs were ultrafiltered to remove unbound small molecule modifiers, and placed in buffer solutions of different pH values (pH4.0, 5.0, 6.0, 7.0, 8.0, 9.0 and 10.0 ) at 25°C for 16 hours to measure the enzyme activity, and calculate the relative activity by taking the enzyme activity of the free enzyme or the modified enzyme under the optimum pH condition as 100% to calculate the relative activity to determine the pH stability of the enzyme. The results are shown in Table 3.
[0036] Table 3 The pH stability of different reaction pH conditions modifying enzymes
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com