Method for preparing lithium ammonium sulfate nonlinear optical crystals and application thereof
A nonlinear optics, lithium ammonium sulfate technology, applied in nonlinear optics, chemical instruments and methods, optics, etc., can solve the problems of serious layered growth habit, high toxicity of raw materials, long growth cycle, etc. The effect of low toxicity and short growth cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
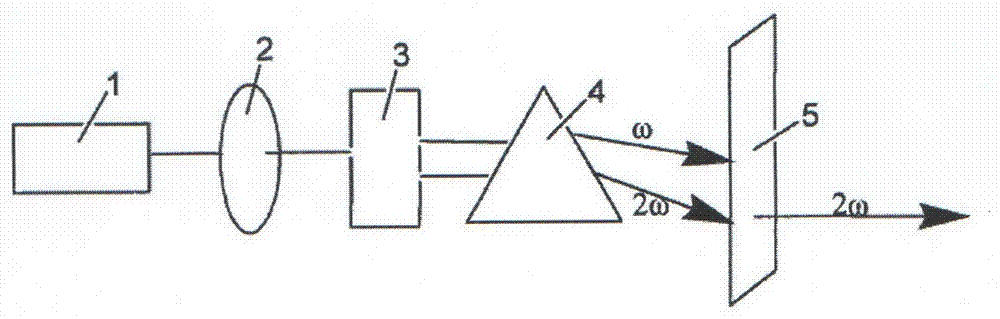
Image
Examples
Embodiment 1
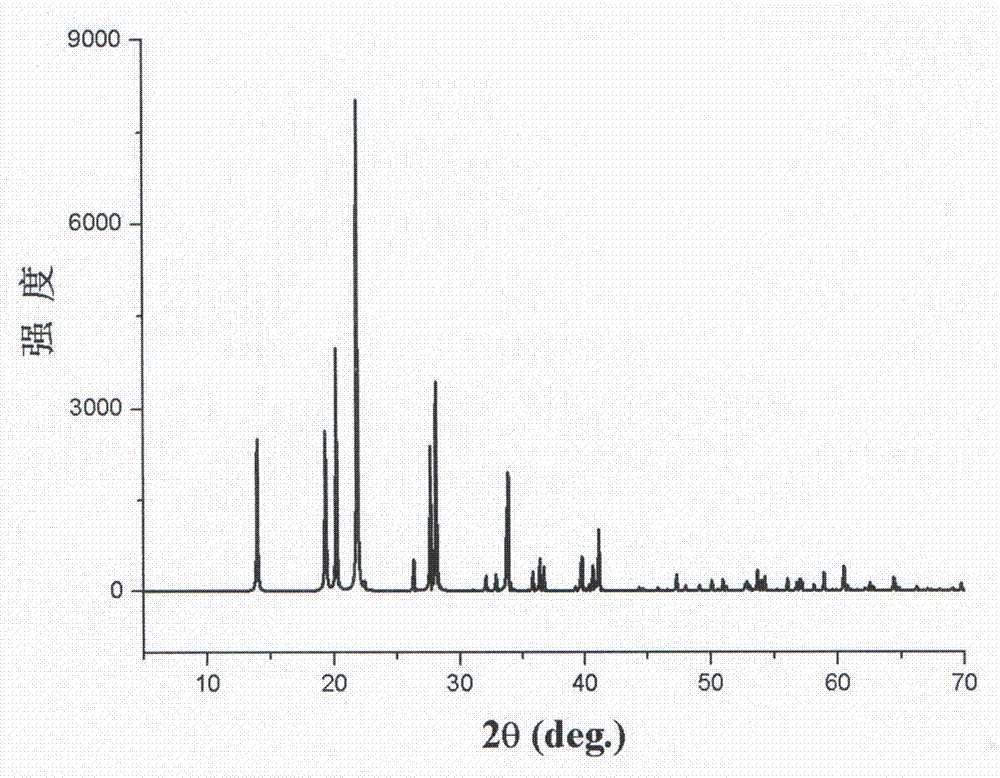
[0028] By chemical reaction formula: Li 2 SO 4 ·H 2 O+(NH 4 ) 2 SO 4 =Li(NH 4 ) SO 4 +H 2 O synthetic lithium ammonium sulfate nonlinear optical crystal;
[0029] a. Will Li 2 SO 4 ·H 2 O and (NH 4 ) 2 SO 4 The elements lithium, ammonium and sulfur in the solution were weighed in a molar ratio of 1:1:1 and put into a clean Erlenmeyer flask (the Erlenmeyer flask must be cleaned with acid first, and then rinsed with deionized water ), add 20ml of deionized water, and then ultrasonic treatment for about 10 minutes to make it fully mixed and dissolved;
[0030] b. Seal the Erlenmeyer flask containing the solution in step a with weighing paper, place it in a static environment without shaking, pollution, and air convection, and puncture the seal with several small holes to adjust the evaporation of water in the aqueous solution. Rate, stand at room temperature for 15 days;
[0031] c. Waiting for the solution in step b to grow crystal particles at the bottom of the ...
Embodiment 2
[0035] By chemical reaction formula: Li 2 SO 4 ·H 2 O+(NH 4 ) 2 SO 4 =Li(NH 4 ) SO 4 +H 2 O synthetic lithium ammonium sulfate nonlinear optical crystal;
[0036] a. Will Li 2 SO 4 ·H 2 O and (NH 4 ) 2 SO 4 The elements lithium, ammonium and sulfur in the solution were weighed in a molar ratio of 1:1:1 and put into a clean Erlenmeyer flask (the Erlenmeyer flask must be cleaned with acid first, and then rinsed with deionized water ), add 25ml of deionized water, and then ultrasonic treatment for 20 minutes to make it fully mixed and dissolved;
[0037] b. Seal the Erlenmeyer flask containing the solution in step a with weighing paper, place it in a static environment without shaking, pollution, and air convection, and puncture the seal with several small holes to adjust the evaporation of water in the aqueous solution. Rate, stand at room temperature for 20 days;
[0038] c. Waiting for the solution in step b to grow crystal particles at the bottom of the Erlenm...
Embodiment 3
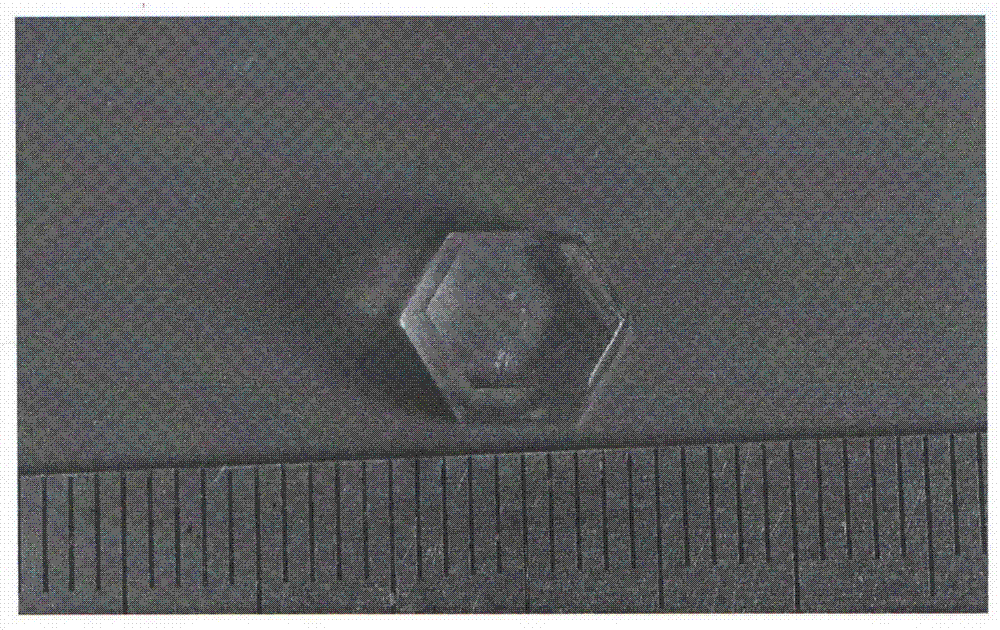
[0042] By chemical reaction formula: Li 2 SO 4 ·H 2 O+(NH 4 ) 2 SO 4 =Li(NH 4 ) SO 4 +H 2 O synthesis of large-size lithium ammonium sulfate nonlinear optical crystals;
[0043] a. Will Li 2 SO 4 ·H 2 O and (NH 4 ) 2 SO 4 The elements lithium, ammonium and sulfur in the solution were weighed in a molar ratio of 1:1:1 and put into a clean Erlenmeyer flask (the Erlenmeyer flask must be cleaned with acid first, and then rinsed with deionized water ), add 30ml of deionized water, and then ultrasonic treatment for 30 minutes to make it fully mixed and dissolved;
[0044] b, seal the Erlenmeyer flask with solution in step a with plastic wrap, place it in a static environment without shaking, pollution, and air convection, and pierce the seal with some small holes to adjust the evaporation rate of water in the aqueous solution, Stand at room temperature for 30 days;
[0045] c. Waiting for the solution in step b to grow crystal particles at the bottom of the Erlenmeye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com