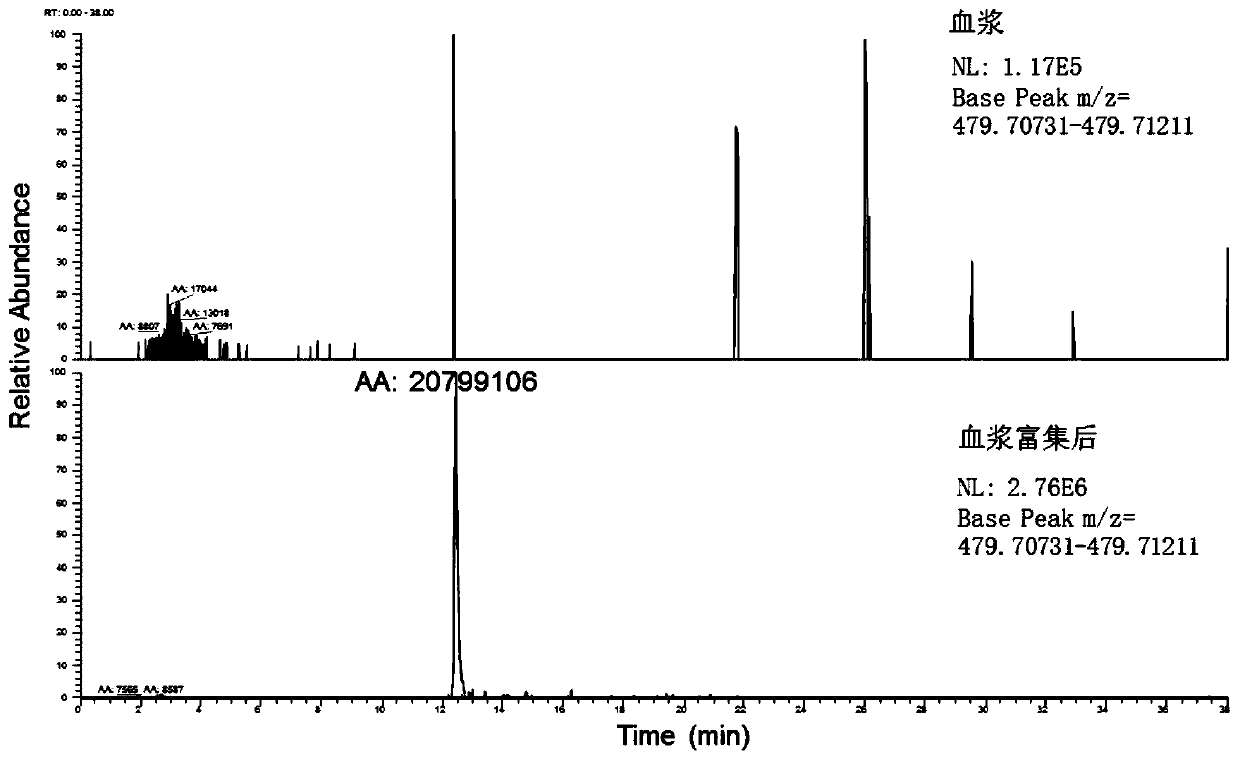
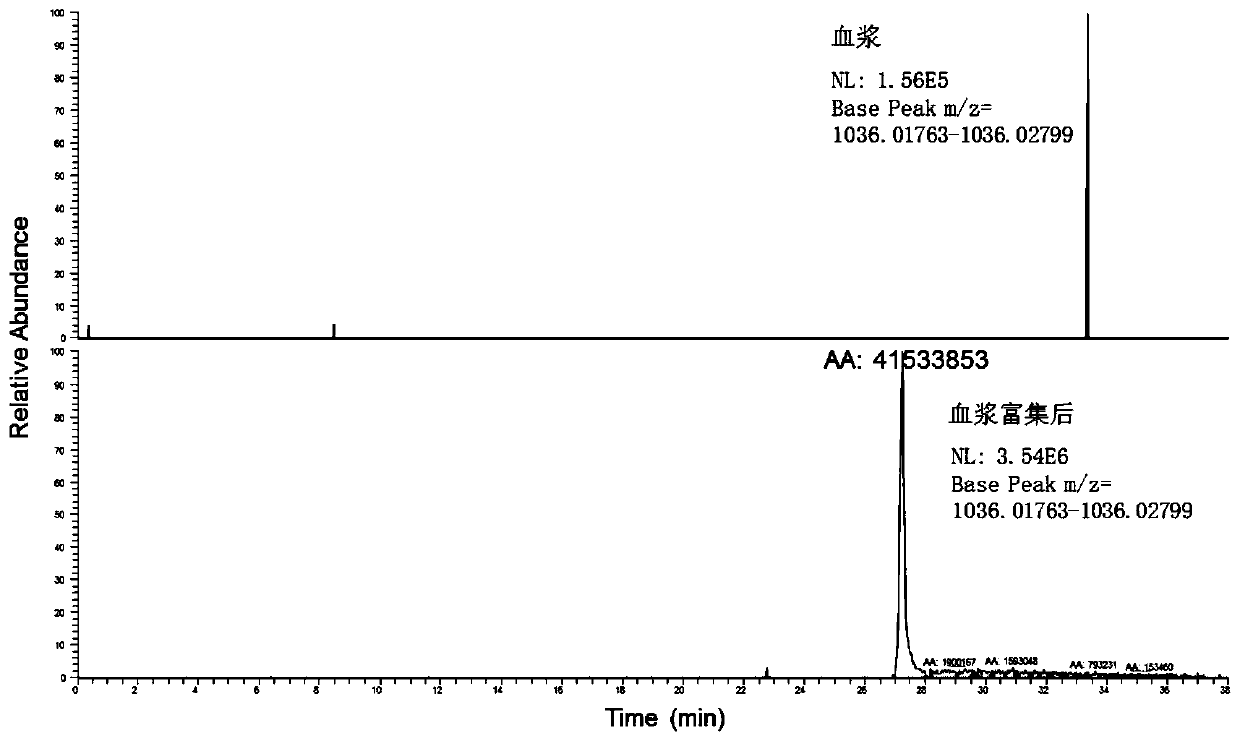
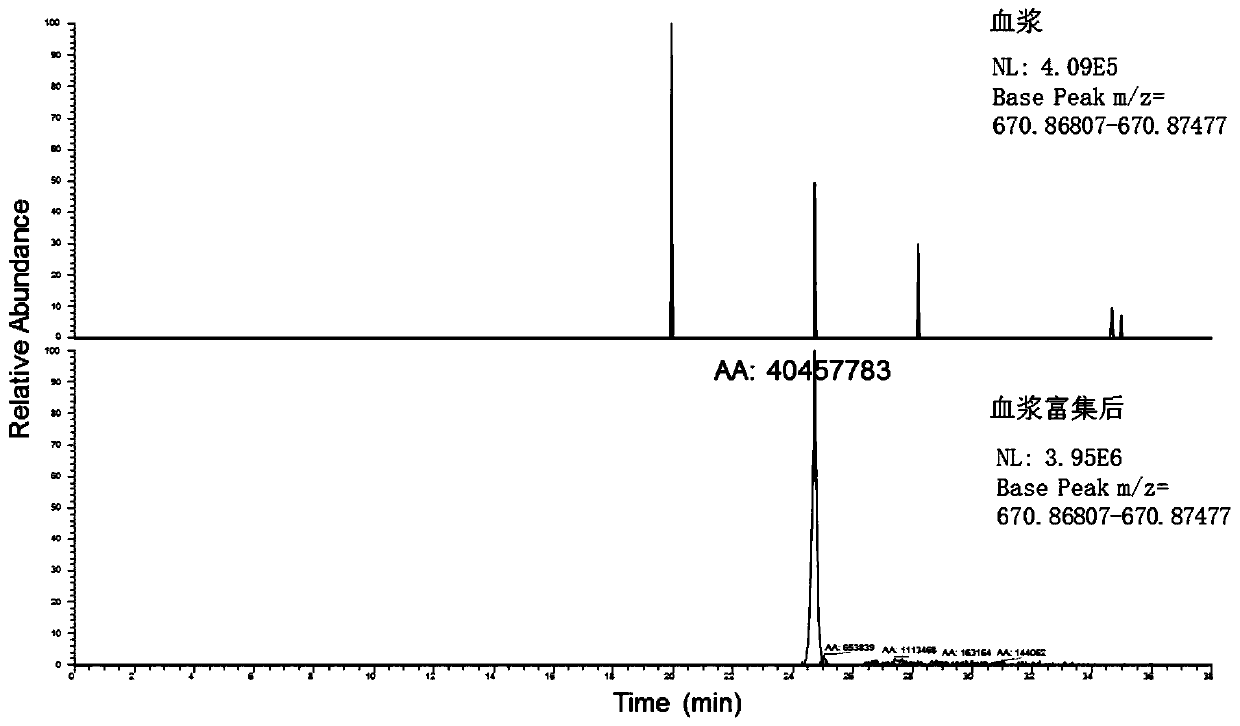
A method for enriching 5 low-abundance proteins in plasma or serum
A low-abundance protein and plasma technology, applied in the field of low-abundance protein enrichment, can solve the problems of high price, unstable measurement results, huge differences, etc. deterministic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Enrichment of 5 low-abundance proteins in the plasma of Example 1
[0051] (1) Preparing the chromatographic column
[0052] Weigh the required phosphocellulose powder as column material (0.5 g column material / 1 sample × number of samples), and distribute it to multiple 50 ml centrifuge tubes (each centrifuge tube contains 1 g column material), And each centrifuge tube was processed as follows:
[0053] ① Add 50 ml of a solution containing 0.05 mol / L sodium hydroxide and 0.5 mol / L sodium chloride to the centrifuge tube, shake to mix, soak for 5 minutes;
[0054] ②Put the centrifuge tube obtained in step ① into a centrifuge and centrifuge at 1000g for 1 minute, then pour off the supernatant;
[0055] ③ Add 50 ml of ultrapure water to the centrifuge tube obtained in step ②, suspend the column material, put it into a centrifuge and centrifuge at 1000g for 1 minute, measure the pH value of the supernatant, pour off the supernatant, if the pH value is less than 10, it can ...
Embodiment 2
[0091] Enrichment of 5 low-abundance proteins in the serum of Example 2
[0092] Serum samples were used instead of plasma samples in Example 1, and other conditions were exactly the same as in Example 1 to enrich the samples.
[0093] Then, in the same method as in Example 1, the enriched sample (ie, the eluted component) was detected by high performance liquid chromatography combined with high resolution mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com