Azithromycin dry suspension and preparation method thereof
A technology for azithromycin and dry suspension, which is applied in the field of azithromycin dry suspension and its preparation, which can solve the problems of affecting drug efficacy, children prone to nausea, and children's difficulty in taking medicine, so as to improve the drug's effective rate and granulate Better effect, excellent effect of alkalinity and sedimentation volume ratio and other indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
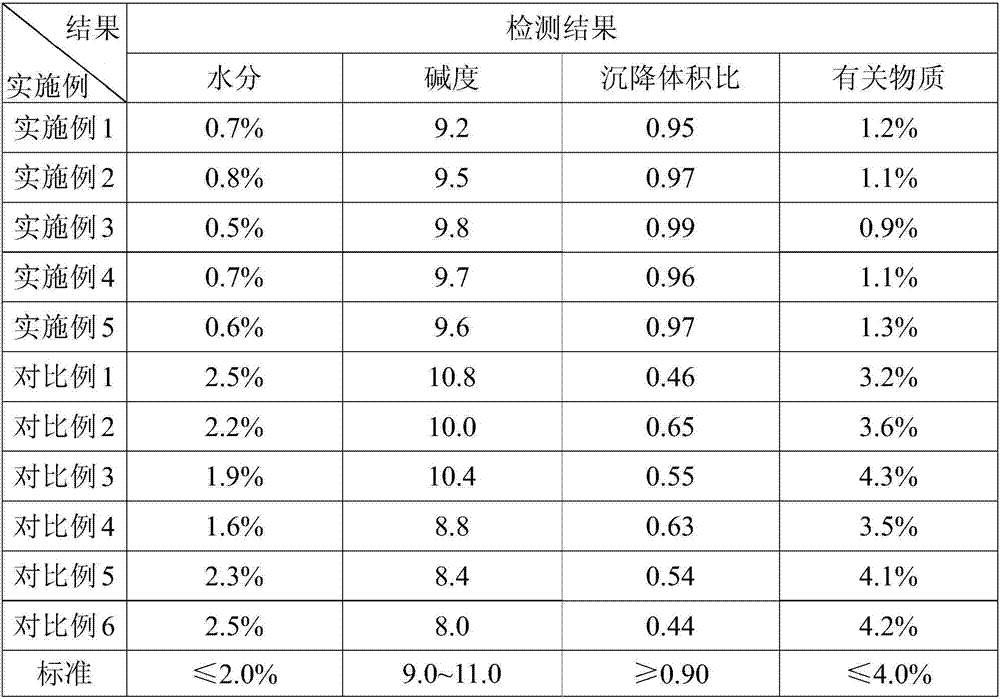
Examples
Embodiment 1
[0022] A dry suspension of azithromycin, comprising the following raw materials: 100 parts of azithromycin, 55 parts of sodium citrate, 85 parts of sodium carboxymethylcellulose, 1700 parts of powdered sugar and 24 parts of sweet orange essence;
[0023] Among them, azithromycin is the main drug, sodium citrate is the flocculant, sodium carboxymethylcellulose is the suspending agent, powdered sugar is the suspending agent and flavoring agent, and sweet orange essence is the aromatic agent.
[0024] The preparation method of above-mentioned azithromycin dry suspension comprises the following steps:
[0025] (1) Crushing and sieving: Azithromycin, sodium citrate, sodium carboxymethylcellulose, and powdered sugar are respectively added into the hopper of the universal pulverizer to pulverize, and then pass through an 80-mesh sieve after pulverization;
[0026] (2) Granulation: add the sieved raw materials in step (1) into the high-efficiency wet granulator and mix them evenly, ad...
Embodiment 2
[0030] A dry suspension of azithromycin comprising the following raw materials: 100 parts of azithromycin, 65 parts of sodium citrate, 95 parts of sodium carboxymethylcellulose, 1750 parts of powdered sugar and 28 parts of sweet orange essence;
[0031] Among them, azithromycin is the main drug, sodium citrate is the flocculant, sodium carboxymethylcellulose is the suspending agent, powdered sugar is the suspending agent and flavoring agent, and sweet orange essence is the aromatic agent.
[0032] The preparation method of above-mentioned azithromycin dry suspension comprises the following steps:
[0033] (1) Crushing and sieving: Azithromycin, sodium citrate, sodium carboxymethylcellulose, and powdered sugar are respectively added into the hopper of the universal pulverizer to pulverize, and then pass through an 80-mesh sieve after pulverization;
[0034] (2) Granulation: add the sieved raw materials in step (1) into the high-efficiency wet granulator and mix them evenly, add...
Embodiment 3
[0038] A dry suspension of azithromycin comprising the following raw materials: 100 parts of azithromycin, 60 parts of sodium citrate, 90 parts of sodium carboxymethylcellulose, 1724 parts of powdered sugar and 26 parts of sweet orange essence;
[0039] Among them, azithromycin is the main drug, sodium citrate is the flocculant, sodium carboxymethylcellulose is the suspending agent, powdered sugar is the suspending agent and flavoring agent, and sweet orange essence is the aromatic agent.
[0040] The preparation method of above-mentioned azithromycin dry suspension comprises the following steps:
[0041] (1) Crushing and sieving: Azithromycin, sodium citrate, sodium carboxymethylcellulose, and powdered sugar are respectively added into the hopper of the universal pulverizer to pulverize, and then pass through an 80-mesh sieve after pulverization;
[0042] (2) Granulation: add the sieved raw materials in step (1) into the high-efficiency wet granulator and mix them evenly, add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com