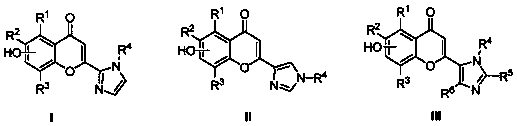
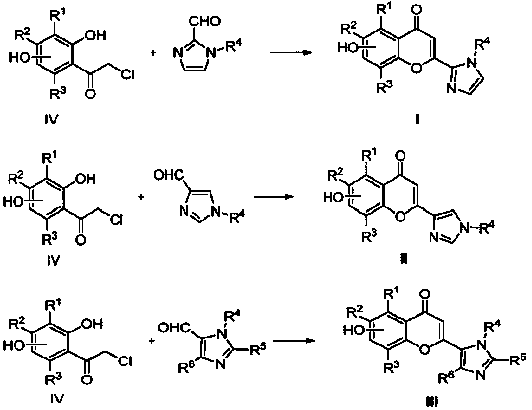
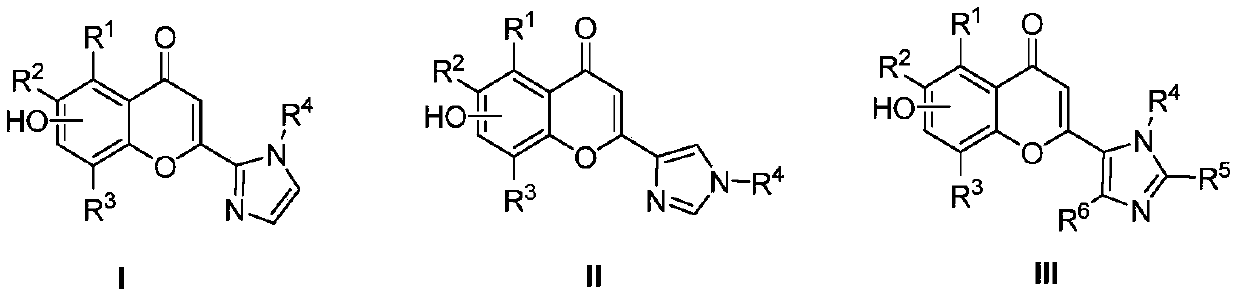
Flavonoid imidazole compounds and preparation method thereof
A technology of flavonoid imidazoles and compounds, applied in the field of organic drug synthesis, to achieve the effects of easy-to-obtain raw materials, simple preparation methods, and short synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the preparation of compound IV-1
[0035]
[0036] According to literature method steps, weigh resorcinol (4.37g, 39.7mmol), ZnCl 2 (0.54g, 39.7mmol), chloroacetonitrile (2.5mL, 39.7mmol) were placed in a three-necked flask, dissolved with ether solution, in an ice-water bath, fed with HCl gas for 4 hours and left to stand overnight, the next day HCl gas was bubbled again for 1 hour, and the white precipitated solid was filtered off. The obtained white solid was then refluxed in 1 mol / L hydrochloric acid solution for 1 hour, cooled in an ice-water bath to condense a large amount of white cotton flocculent solid, filtered by suction and dried to obtain 6.83 g of compound IV-1. Light pink powdery solid with a melting point of 130-132°C and a yield of 92.5%.
Embodiment 2
[0037] Embodiment 2, the preparation of compound I-1
[0038]
[0039] Add 10% NaOH (7mL) dropwise to a 100mL round bottom flask containing compound IV-1 (0.93g, 5.0mmol), 2-imidazolaldehyde (0.48g, 5.0mmol) and an appropriate amount of ethanol (15mL), and stir at room temperature After 24 hours of reaction, TLC traced until the reaction was completed, neutralized it to pH = 7 with 1 mol / L dilute hydrochloric acid, recrystallized and dried to obtain 0.98 g of compound I-1 with a yield of 86.9%.
[0040] Compound I-1: yellow powder; melting point 157-158°C; 1 H NMR (400MHz, DMSO-d 6 ) δ:12.39(s,1H,OH),7.61(d,J=8.4Hz,1H,flavone-5-H),7.33(m,2H,imidazole-4,5-2H),6.79(s,1H , flavone-3-H), 6.71 (d, J=8.4Hz, 1H, flavone-6-H), 6.60 (s, 1H, flavone-8-H) ppm.
Embodiment 3
[0041] Embodiment 3, the preparation of compound 1-2
[0042]
[0043] Add 10% NaOH dropwise to a 100mL round bottom flask containing compound IV-1 (0.93g, 5.0mmol), N-n-propyl-2-imidazolaldehyde (0.69g, 5.0mmol) and an appropriate amount of ethanol (15mL). (7 mL), stirred and reacted at room temperature for 24 hours, followed by thin-layer chromatography until the end of the reaction, neutralized to pH=7 with 1mol / L dilute hydrochloric acid, and then obtained 1.11g of compound I-2 after recrystallization and drying. was 82.7%.
[0044] Compound I-2: yellow powder; melting point 161-162°C; 1 H NMR (400MHz, DMSO-d 6 ) δ: 7.61(d, J=8.5Hz, 1H, flavone-5-H), 7.43(d, J=0.9Hz, 1H, imidazole-4-H), 7.22(d, J=0.7Hz, 1H, imidazole-5-H),6.78–6.63(m,3H,flavone-3-H, flavone-6-H,flavone-8-H),4.15(t,J=7.1Hz,2H,imidazole-CH 2 ), 1.72 (dd, J = 14.4, 7.3Hz, 2H, CH 2 ), 0.85(t, J=7.4Hz, 3H, CH 3 ) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com