Engineering super-hydrophobic metal coating and preparation method thereof
A metal coating, super-hydrophobic technology, applied in the field of new super-hydrophobic metal coating and its preparation, can solve problems such as difficulty in guaranteeing service life, poor organic compound chemistry, thermodynamics and mechanical stability, etc., and achieves stable and controllable process. Excellent corrosion resistance, the effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In the present embodiment, 260g nickel sulfate, 360g sodium citrate, 33g copper sulfate, 204g sodium bromide, 260g ammonium chloride, 980g sodium tungstate, and 10g glycine are added to deionized water to prepare a 10L plating solution, and The pH of the bath was adjusted to 8 with sulfuric acid and ammonia.
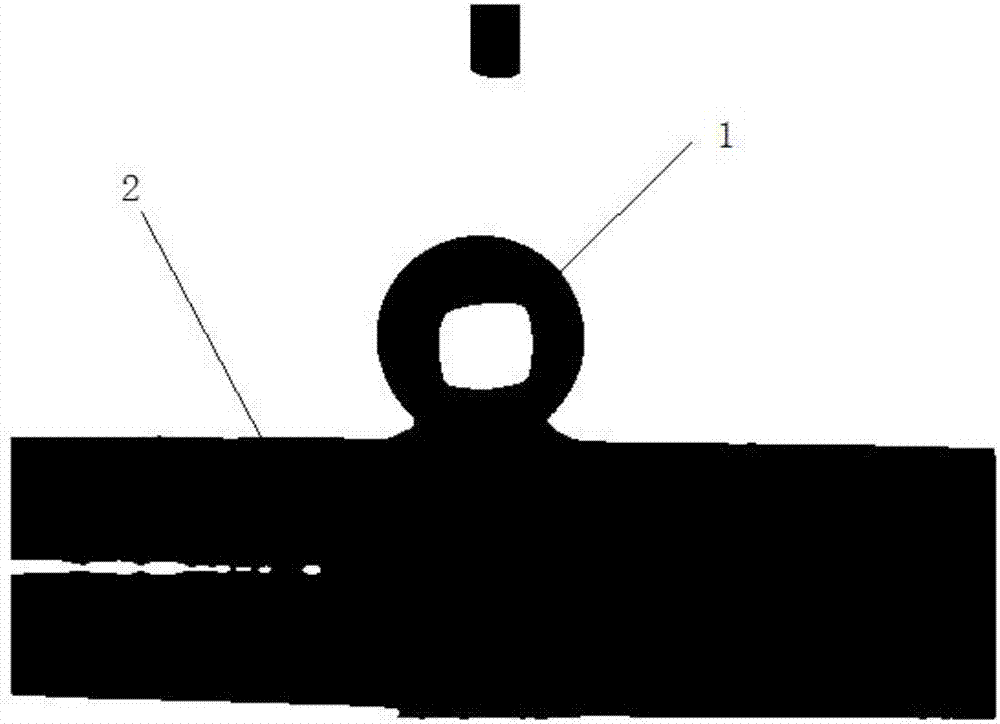
[0027] In this embodiment, low-carbon steel is used as the cathode substrate, and electroplating is performed by direct current electrodeposition after polishing and cleaning, and the cathode current density is 4A / dm 2 , Plating time is 60 minutes. Such as figure 1 As shown, the deposited surface coating 2 exhibits superhydrophobic properties after being cleaned and dried, and the contact angle of the water droplet 1 on its surface is 153±2°.
Embodiment 2
[0029] In the present embodiment, use 290g of nickel sulfate, 320g of sodium citrate, 44g of copper sulfate, 180g of sodium bromide, 200g of ammonium chloride, 980g of sodium tungstate and 15g of glycine to be added to deionized water to prepare a 10L plating solution, and pass Sulfuric acid and ammonia adjust the pH of the bath to 8.
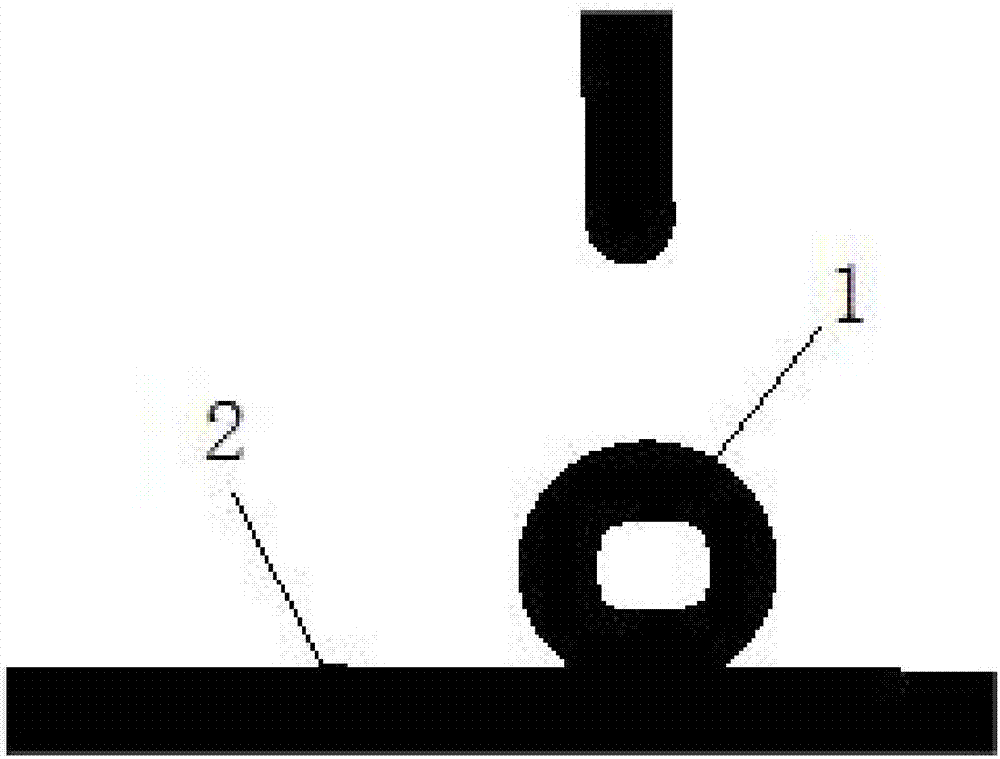
[0030] In this example, red copper is used as the cathode substrate, and electroplating is performed by pulse electrodeposition after polishing and cleaning, and the cathode current density is 5A / dm 2 , the duty cycle is 0.6, and the plating time is 90 minutes. Such as figure 2 As shown, the deposited surface coating 2 exhibits superhydrophobic properties after being cleaned and dried, and the contact angle of the water droplet 1 on its surface is 151±2°.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com