Injectable hydrogel and its preparation and application
A technology for injecting water and hydrogel, which is applied in the direction of non-active ingredient medical preparations, prostheses, aerosol delivery, etc., to achieve the effect of easy adjustment, overcoming safety hazards, and controllable slow-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
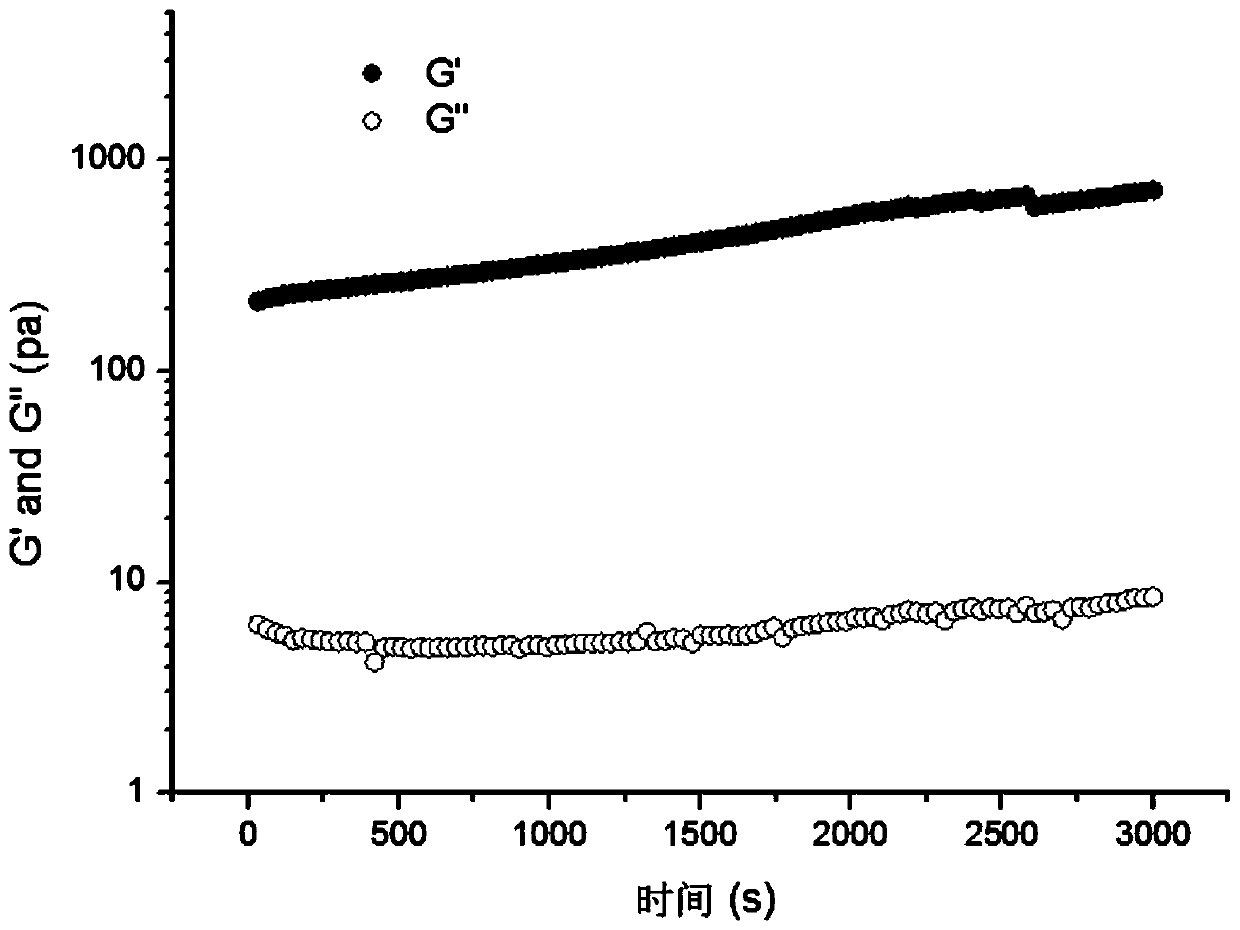
[0035]Add 0.5 g of butyraldehyde-polyethylene glycol-butyraldehyde (m=100) and 1 g of four-arm terminal amino-modified polyethylene glycol (n=1000, Laysan) to 28.5 g of sterile deionized water, and shake rapidly at 37°C Mix well, and after 35 seconds, 30 g of injectable hydrogel in a transparent and uniform state are obtained.
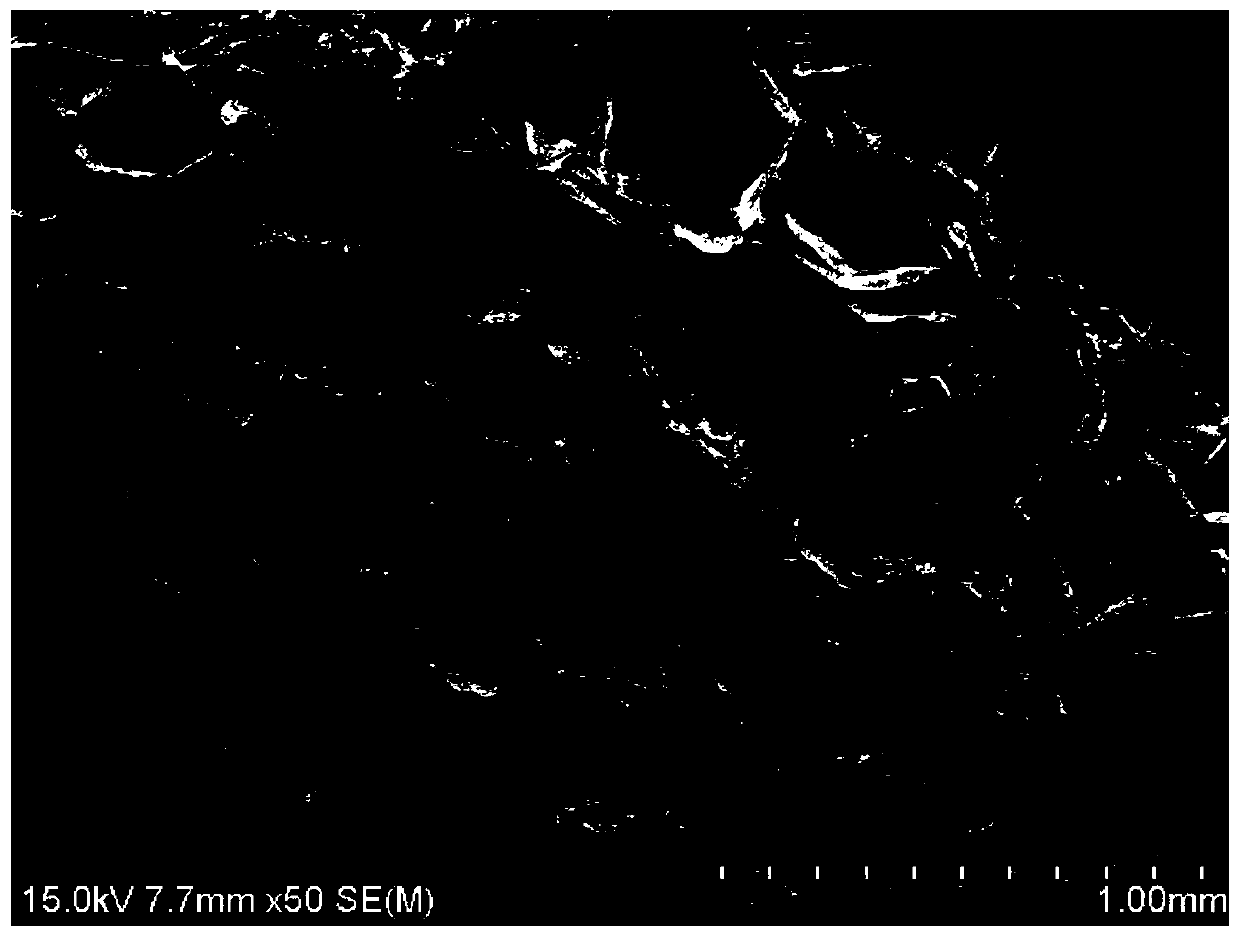
[0036] The scanning electron microscope image of the injectable hydrogel is shown in figure 1 , showing that the hydrogel has a porous and dense structure with a pore size of 0.1mm-0.4mm.
Embodiment 2
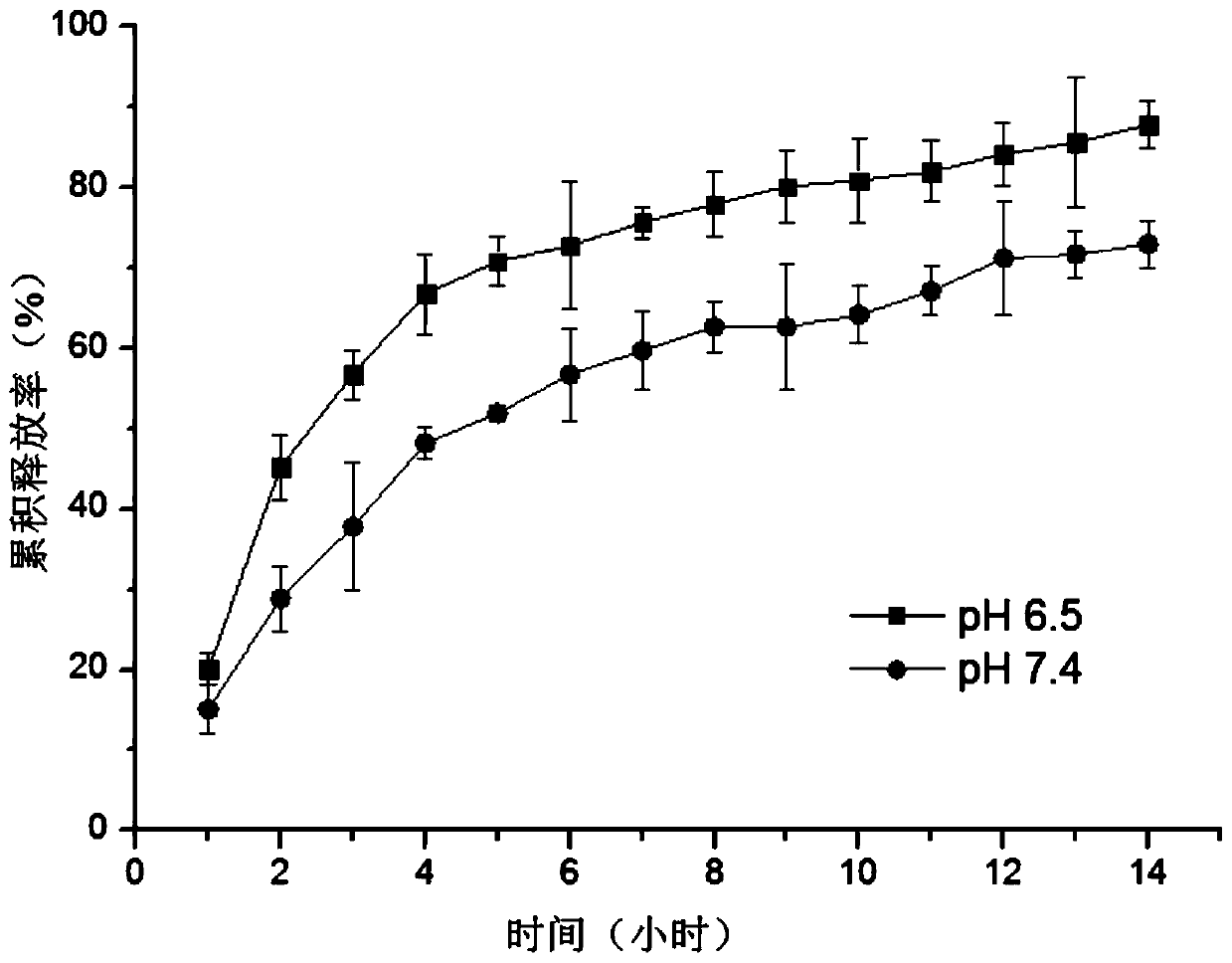
[0038] Add 5g of butyraldehyde-polyethylene glycol-butyraldehyde (m=16000) and 1g of four-arm terminal amino-modified polyethylene glycol (n=5000, Laysan) to add 34g of disodium hydrogen phosphate-citric acid buffer (pH= 6.5), at 37°C, oscillate quickly and mix evenly, and after 30 seconds, 40 g of injectable hydrogel in a transparent and uniform state is obtained.
[0039] The scanning electron microscope image of the obtained injectable hydrogel shows that the hydrogel has a porous and dense structure with a pore diameter of 0.1mm-0.3mm.
Embodiment 3
[0041] 14g butyraldehyde-polyethylene glycol-butyraldehyde (m=10000) and polyethylene glycol (n=5700, Laysan) of 1g four-arm terminal amino-modification are added 85g disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution ( pH=7.4), 37° C., oscillating rapidly and mixing evenly, and after 25 seconds, 100 g of injectable hydrogel in a transparent and uniform state was obtained.
[0042] The scanning electron microscope image of the obtained injectable hydrogel shows that the hydrogel has a porous and dense structure with a pore diameter of 0.1mm-0.3mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com