Preparation method of cycloxaprid
A technology of cyclopyridin and chloromethylpyridine, which is applied in the direction of organic chemistry, can solve the problem that the yield is only 55%, and achieve the effect of ensuring the purity and safety of the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
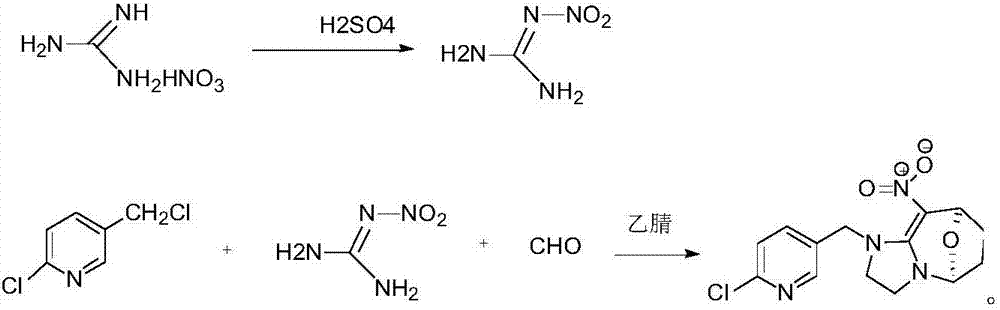
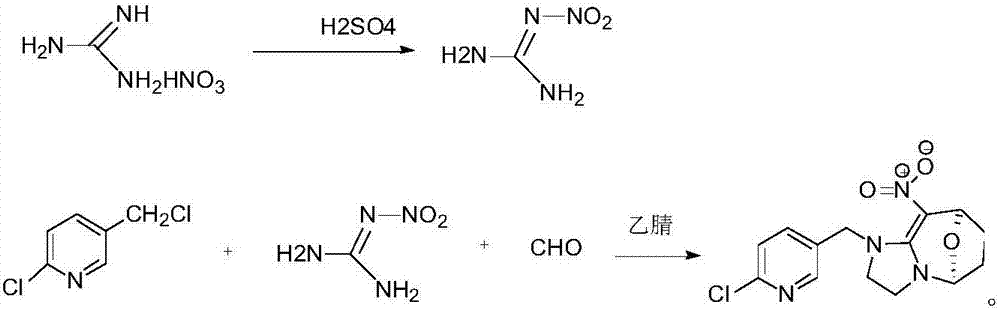
[0020] Add 100g concentrated sulfuric acid in 500ml round bottom flask, 50g guanidine nitrate is warmed up to 40 ℃ and reacts for 30min, puts into 400ml ice water and freezes and filters to obtain 68g nitroguanidine that the mass concentration percentage is 30% moisture; In 500ml round bottom flask Add 50g of solvent acetonitrile, 60g of paraformaldehyde, 68g of nitroguanidine containing 30% water in mass concentration, start stirring and heat up to 80°C, add 120g of 50% 2-chloro-5- Chloromethylpyridine solution, control the temperature not to exceed 80°C, slowly add 2-chloro-5-chloromethylpyridine dropwise, after the dropwise addition, control the temperature at 80°C and keep it warm for 10 hours, after the heat preservation, cool down to 10°C, Filter and dry to obtain cyclopyridin solid.
[0021] In this example, the content of cycloxaprid was analyzed by liquid chromatography to be 98.2%, and the molar yield of cyclopyrid was 95%.
Embodiment 2
[0023] Add 120g concentrated sulfuric acid in 500ml round bottom flask, 60g guanidine nitrate is heated up to 42 ℃ and reacts 40min, puts into 400ml ice water and freezes and filters to obtain the 81.6g nitroguanidine that contains mass concentration percentage and is 30% moisture; In 50ml round bottom flask Add 60g of solvent acetonitrile, 72g of paraformaldehyde, 81.6g of nitroguanidine containing 30% moisture in mass concentration, start stirring and heat up to 85°C, add 127g of 50% 2-chloro- 5-chloromethylpyridine solution, control the temperature not to exceed 85°C, slowly add 2-chloro-5-chloromethylpyridine dropwise, after the dropwise addition, control the temperature at 85°C for 8 hours, and cool down to 10 °C, filtered, and dried to obtain cyclopyridin as a solid.
[0024] In this example, the content of cycloxaprid was analyzed by liquid chromatography to be 98.6%, and the molar yield of cyclopyrid was 95.2%.
Embodiment 3
[0026] Add 100g concentrated sulfuric acid in 500ml round bottom flask, 50g guanidine nitrate is warmed up to 41 ℃ and reacts for 30min, put into 400ml ice water and freeze and filter to obtain 68g nitroguanidine that the mass concentration percentage is 30% moisture; In 500ml round bottom flask Add 50g of solvent acetonitrile, 60g of paraformaldehyde, 68g of nitroguanidine containing 30% water in mass concentration, start stirring and heat up to 80°C, add 120g of 50% 2-chloro-5- Chloromethylpyridine solution, control the temperature not to exceed 80°C, slowly add 2-chloro-5-chloromethylpyridine dropwise, after the dropwise addition, control the temperature at 85°C for 9 hours, and after the heat preservation, cool down to 10°C, Filter and dry to obtain cyclopyridin solid.
[0027] In this example, the content of cycloxaprid was analyzed by liquid chromatography to be 98.8%, and the molar yield of cyclopyrid was 95.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com