Biphenyl aromatic hydrocarbon, water-soluble biphenyl aromatic hydrocarbon and preparation method thereof
A biphenyl aromatic hydrocarbon, water-soluble technology, applied in the field of biphenyl aromatic hydrocarbon, water-soluble biphenyl aromatic hydrocarbon and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
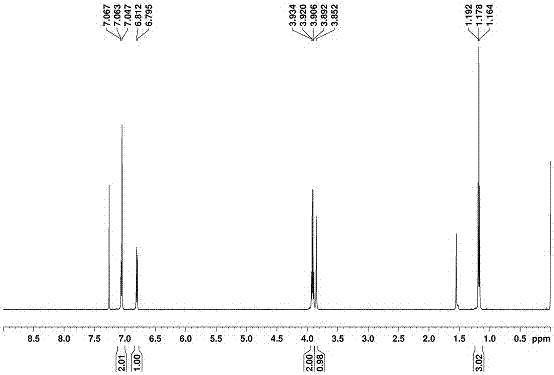
[0058] The preparation and characterization of embodiment 1 novel biphenyl aromatics:
[0059] Dissolve 2,2'-ethoxybiphenyl:paraformaldehyde:boron trifluoride ether in 1,2-dichloroethane in a molar ratio of 1:1.2:1~1:2:2. The mixture was stirred at room temperature for 0.5h, and the reaction solution gradually changed from colorless to light red until wine red. During the reaction, it was detected by spot plate, and quenched with 50mL of water after 0.5h. The whole reaction was carried out at room temperature. The organic phase was sequentially washed with saturated NaHCO 3 , saturated NaCl solution was washed and extracted, and then the organic phase was washed with anhydrous Na 2 SO 4 Dry treatment. Then carry out the sample mixing and spin-drying to pack the column. Through column chromatography, the volume ratio of ethyl acetate and petroleum ether as the developing solvent was adjusted gradually from 1:10 to 1:5 and passed through the column to obtain 2,2'-ethoxybiphe...
Embodiment 2
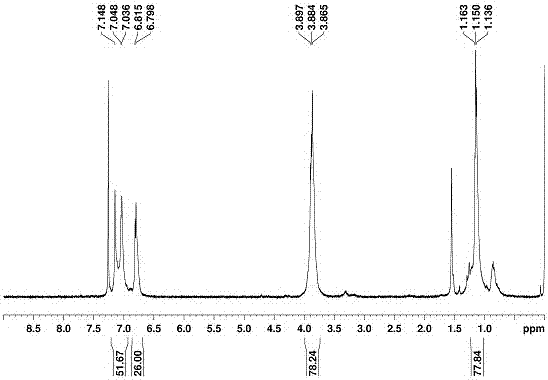
[0060] The preparation of embodiment 2 novel water-soluble biphenyl aromatic hydrocarbons:
[0061] The following preparation of water-soluble biphenyl aromatic hydrocarbons is based on water-soluble biphenyl [5] aromatic hydrocarbons as an example, and the preparation methods of other water-soluble biphenyl aromatic hydrocarbons are similar to water-soluble biphenyl [5] aromatic hydrocarbons, and the specific steps are:
[0062] 1,2,2'-Perhydroxybiphenyl[5]arene (OH-BP5).
[0063] The novel biphenyl[5]arene (500 mg, 0.4 mmol) was dissolved in 30 ml of anhydrous dichloromethane and an excess of 1 mL (10 mol) of BBr was carefully added 3 (large overdose), in N 2 Under atmosphere and stirring for 12h. After the reaction was completed, we slowly added the reaction solution dropwise to the ice-water mixture, and a large amount of precipitates were precipitated. The target product can be obtained by suction filtration under reduced pressure and then washing with a large amount o...
Embodiment 3
[0070] Example 3: Preparation and characterization of spermidine and CBP5 inclusion complex:
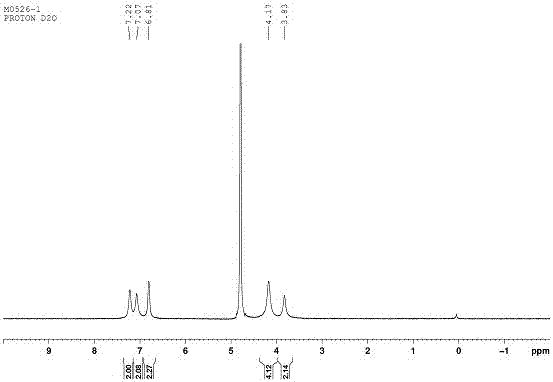
[0071] Accurately weigh 7.4 mg of spermidine (0.05 mmol) and 80 mg of CP6A (0.05 mmol) and mix them together, dissolve them in 10 mL of water, and mix evenly. The filtrate is vacuum freeze-dried to obtain the inclusion compound of spermidine and CBP5. At the same time we formulated the bulk CBP (2mM) alone and spermidine alone (2mM) in D 2 O, through 1 H-NMR shows that spermidine is encapsulated in the host CBP5 as in Figure 15-16 .
[0072] Depend on Figure 15 It can be clearly seen that when spermidine is enclosed by CBP5, its chemical shift value changes significantly, and the proton peak broadens significantly and moves to the high field. This indicates that spermidine molecules enter the cavity of CBP5 and produce a shielding effect, which makes the hydrogen spectrum peaks of imine molecules shift to high fields. The broadening of the peak shape indicates that it is compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com