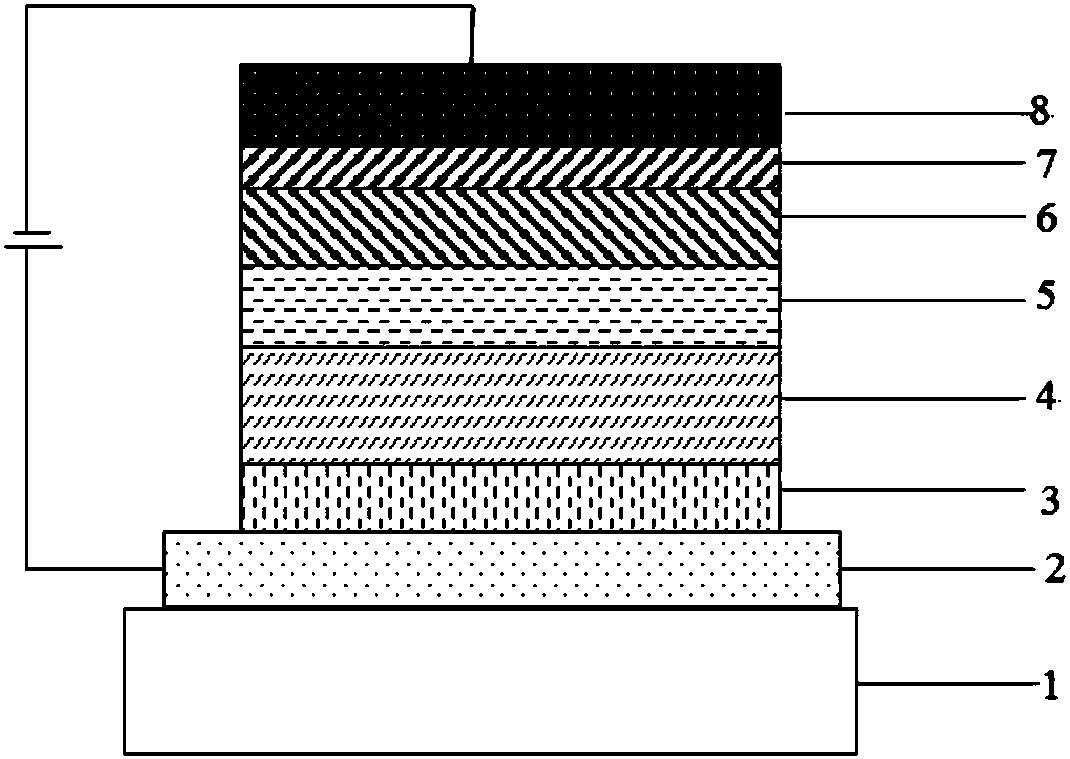
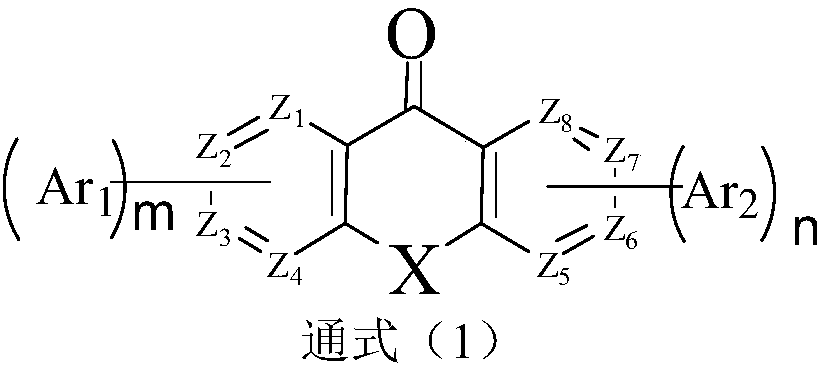
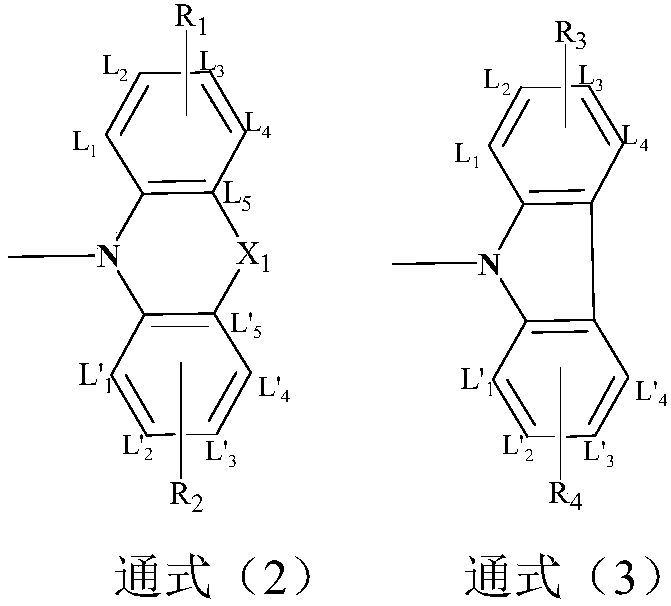
Azaxanthone compound and applications of azaxanthone compound in OLED light emitting devices
A technology of xanthone and compound, applied in the direction of electric solid device, semiconductor device, chemical instrument and method, can solve the problems of limited application, lower triplet energy level, etc., achieve good optoelectronic performance, increase orbital overlap, Avoid the effect of agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the synthesis of R structure (shown in general formula 3)
[0051]
[0052] The preparation method of R structure (shown in general formula 3):
[0053] Weigh raw material I o-bromonitro compound, raw material II boric acid, and dissolve it in a mixed solvent of toluene and ethanol with a volume ratio of 2:1, and add potassium carbonate aqueous solution, Pd(PPh 3 ) 4 , reacted at 95-110°C for 10-24 hours, cooled to and filtered the reaction solution, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product; the molar ratio of o-bromonitro compound to boric acid was 1:1.0~3.0; o-bromo The molar ratio of nitro compound to potassium carbonate is 1:1.0~3.0; o-bromonitro compound and Pd(PPh 3 ) 4 The molar ratio is 1:0.006~0.02;
[0054] Weigh the product from the previous step, dissolve it with o-dichlorobenzene, and then add PPh 3 , under an inert atmosphere, react the mixed solution of the above reactant...
Embodiment 2
[0061] Embodiment 2: the synthesis of R structure (shown in general formula 2)
[0062]
[0063] The preparation method of Reaction Formula 3-1 is: Weigh the corresponding raw material 1-o-aminophenol, raw material 2-o-nitrophenol, and iodine, stir and dissolve them with diethylene glycol as a solvent, and heat to 250-270°C under an inert atmosphere to react 12 -24 hours, take a sample point plate, after the reaction is over, naturally cool to room temperature, there is solid precipitation, filter, take the filter cake and pass through the neutral silica gel column to obtain the intermediate En; the mole of the o-nitrophenol and o-aminophenol The ratio is 1:0.8~2.0; the molar ratio of the o-nitrophenol and iodine is 1:0.05~0.1;
[0064] The preparation method of reaction formula 3-2 is: weigh the corresponding bromide and arylamine with methyl formate, then add Pd 2 (dba) 3 , tri-tert-butylphosphine, sodium tert-butoxide; under an inert atmosphere, react the mixed solutio...
Embodiment 3
[0075] Embodiment 3: the synthesis of compound 1
[0076]
[0077] In a 250ml four-necked flask, add 0.01mol 3-bromo-6-iodo-9-oxa-1-aza-anthracene-10-one, 0.030mol carbazole, 0.03mol tert-butyl Sodium alkoxide, 1 x 10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, reaction complete, natural cooling, filtration, filtrate rotary evaporation, silica gel column to obtain the target product with a purity of 99.12% and a yield of 63.80%. HRMS(m / z): [M+H] + , the theoretical value is 528.1712, and the measured value is 528.1703.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com