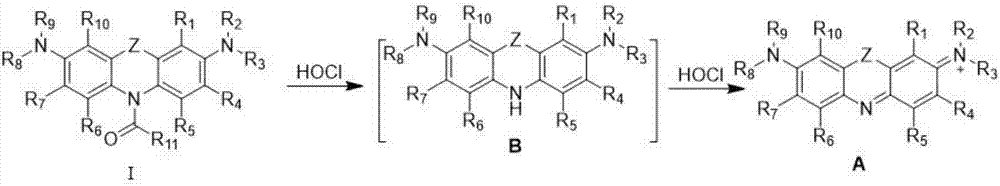
Hypochlorous acid detection fluorescent probes and preparation method and application thereof
A fluorescent probe, hypochlorous acid technology, applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
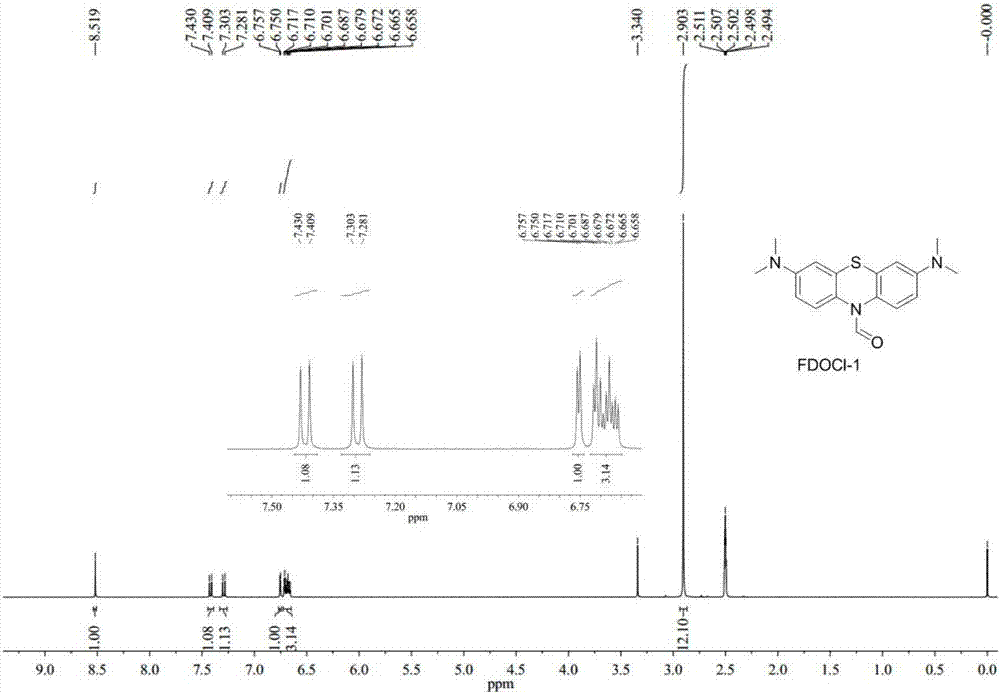
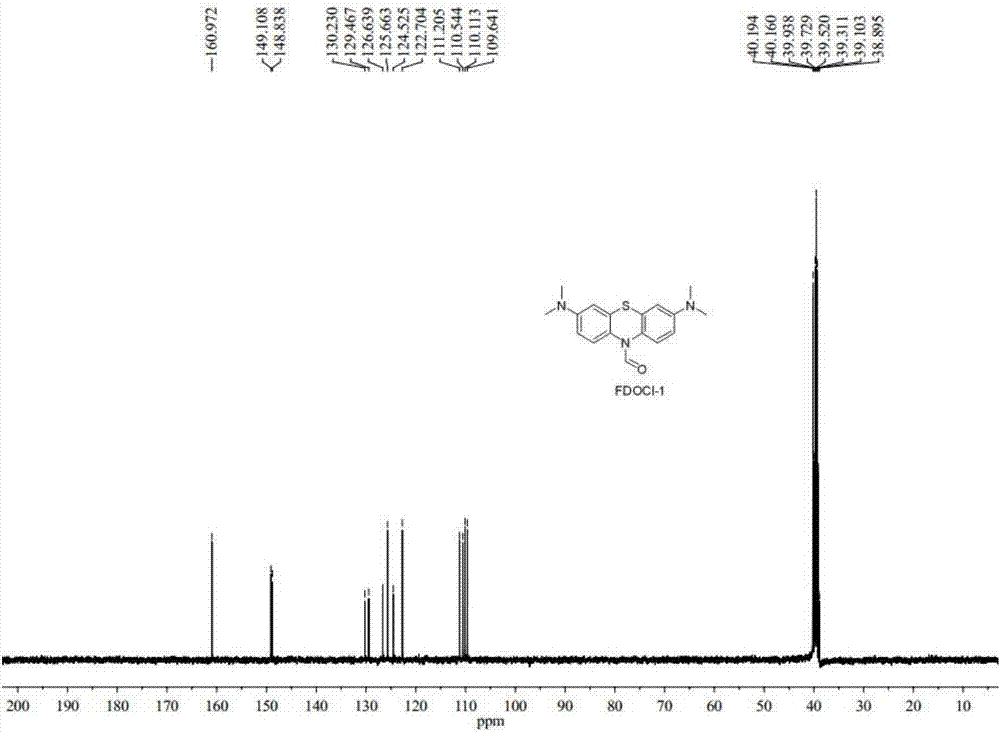
[0048] Preparation of compound FDOCl-1 (R11 is hydrogen) with formula I structure and research on its response performance with hypochlorous acid:
[0049]
[0050] (1) Preparation of Vilsmeyer-Haack Reagent
[0051] Dimethylformamide (4.6g, 62.52mmol) was dissolved in 20ml of dichloromethane, and thionyl chloride (7.44g, 62.52mmol) diluted with 10ml of dichloromethane was added dropwise at room temperature. The reaction was stirred for 15 minutes. After the reaction was completed, the solvent was directly evaporated to dryness, and the obtained Wilsmeyer-Hacker formylating reagent was directly used in the next step reaction.
[0052] (2) Preparation of FDOCl-1
[0053] Add methylene blue (5.0 g, 15.63 mmol) into a three-necked flask and dissolve it with 30 mL of dichloromethane and 40 mL of water. Sodium carbonate (6.63g, 62.52mmol) was added to the system and the system was stirred at 40°C. After nitrogen protection, sodium dithionite (8.16g, 46.89mmol) dissolved in 20mL ...
Embodiment 2
[0064] Preparation of compound FDOCl-2 (R11 is a substituted amino group) with the structure of formula I and research on its response performance with hypochlorous acid
[0065]
[0066] (1) Preparation of compounds
[0067] Add methylene blue (2.0 g, 6.25 mmol) into a three-necked flask and dissolve it with 15 mL of dichloromethane and 40 mL of water. Sodium carbonate (2.65g, 25.00mmol) was added to the system and the system was stirred at 40°C. After nitrogen protection, sodium dithionite (3.26g, 18.75mmol) dissolved in 20mL of water was added dropwise to the system. After the addition was completed, the reaction was carried out for 20 minutes. At this time, the system was clearly separated, and the lower layer was a yellow solution. The system was left to stand, and then 10 mL of triphosgene (0.93 g, 3.13 mmol) dissolved in dichloromethane was added dropwise to the system, and then transferred to room temperature and stirred for 1 h. The system was left to stand, and ...
Embodiment 3
[0072] Preparation of compound FDOCl-3 (R11 is an alkyl group) with the structure of formula I and research on its response performance with hypochlorous acid
[0073]
[0074] (1) Preparation of target compound
[0075] Add methylene blue (2.0 g, 6.25 mmol) into a three-necked flask and dissolve it with 15 mL of dichloromethane and 40 mL of water. Sodium carbonate (2.65g, 25.00mmol) was added to the system and the system was stirred at 40°C. After nitrogen protection, sodium dithionite (3.26g, 18.75mmol) dissolved in 20mL of water was added dropwise to the system. After the addition was completed, the reaction was carried out for 20 minutes. At this time, the system was clearly separated, and the lower layer was a yellow solution. Let the system stand still, after the layers were separated, suck out the lower layer of clear night with a syringe, add 4-dimethylaminopyridine (0.76g, 6.25mmol), sodium carbonate (1.99g, 18.75mmol) and 20mL of dichloromethane mixed system, ice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com