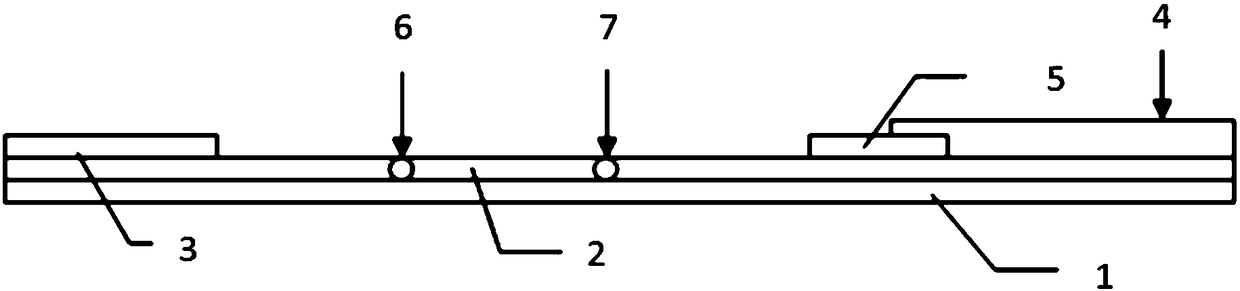
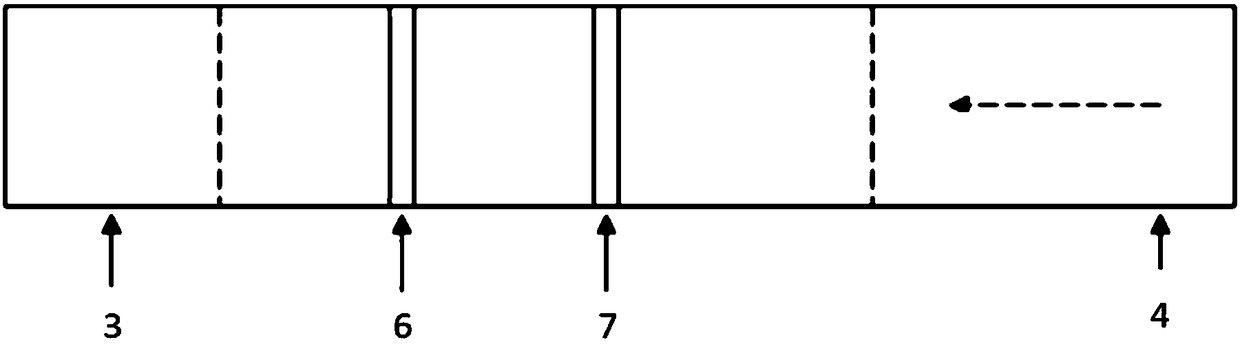
Fluorescent immunochromatographic test paper for quantitative detection of human parathyroid hormone and preparation method thereof
A technique of fluorescence immunochromatography and parathyroid hormone, applied in the field of immunoassay, can solve the problems of low contrast between color bands and background, lack of high specificity detection, and inability to realize quantitative detection, etc., so as to reduce the function of parathyroid glands Decreased incidence, improved detection sensitivity and confidence in results, reduced effects of external conditions and background
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Human PTH epitope peptide
[0042] The human PTH described herein is known in the art, and the complete PTH is composed of a single polypeptide chain containing 84 amino acids, with a molecular weight of about 9500 Daltons. Its amino acid sequence is known in the art and can be found in professional databases such as NCBI. The specific sequence is as follows:
[0043] Human PTH(1-84):
[0044] SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNFVALGAPLAPRDAGSQRPRKKEDNVLVESHEKSLGEADKADVNVLTKAKSQ (SEQ ID NO: 1)
[0045] The inventors of the present invention finally screened and obtained two antigenic epitope peptides with good antigenicity after a lot of theoretical research and experimental exploration.
[0046] PTH epitope peptide (1) is a peptide segment containing 11 amino acids at the 19th-29th position of the N-terminal of the human PTH polypeptide, thereby constituting an antigenic epitope peptide (1) containing 11 amino acids: ERVEWLRKKLQ (SEQ ID NO :2)
[0047] ...
Embodiment 2
[0050] Example 2: Preparation of PTH Antibody
[0051] The PTH epitope peptides (1) and (2) obtained in Example 1 were linked to carrier proteins to prepare antigens (1) and (2) for immunization, and the animals were immunized with the obtained antigens (1) and (2), respectively, Antigen (1) is thus used to prepare specific monoclonal antibodies and polyclonal antibodies, and antigen (2) is used to prepare specific monoclonal antibodies and polyclonal antibodies.
[0052] 1. Antigen preparation: PTH peptides were linked with carrier protein BSA to prepare PTH antigen. Take 0.25 mL each of PBS with pH 6.00.01 mol / L and dimethyl sulfoxide (DMSO) to dissolve 5 mg of BSA, and take 1.2 mg of MBS to dissolve in 100 mL of DMSO. Add MBS to the BSA solution and stir at room temperature for 30 minutes, then centrifuge at 5000 r / min at 4°C to collect the supernatant. Dissolve PTH (19-29) and PTH (51-71) in 0.01mol / L pH7.2 PBS and DMSO respectively in about 500 μL. Mix BSA-MBS with eac...
Embodiment 3
[0066] Example 3: Specific identification of human PTH antibodies (1) and (2)
[0067] Detection was performed by ELISA. The ELISA plates were coated with human PTH, Actin protein, and neuron-specific enolase NSE as detection antigens, and the specific reactions of the prepared PTH monoclonal antibodies (1) and (2) with different proteins were detected by ELISA, respectively. Normal BALB / c mouse serum was used as negative control, and PBS solution was used as blank control.
[0068] Result: PTH monoclonal antibody (1) and (2) only react positively (P / N>2.1) with PTH respectively, and are negative with Actin protein, neuron-specific enolase NSE reaction, illustrate and utilize the present invention The monoclonal antibodies (1) and (2) prepared from the PTH epitope peptide have specificity respectively.
[0069] Identification of polyclonal antibodies was performed using the same method as described above for identifying the specificity of monoclonal antibodies.
[0070] The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com