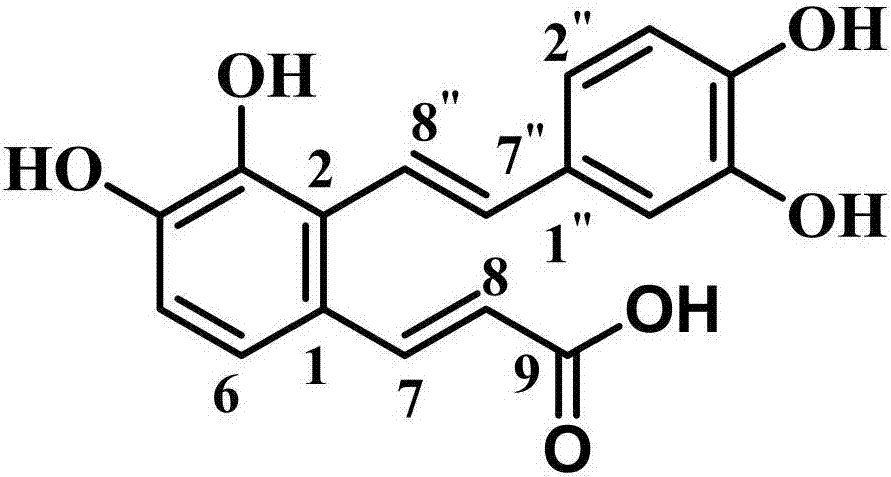
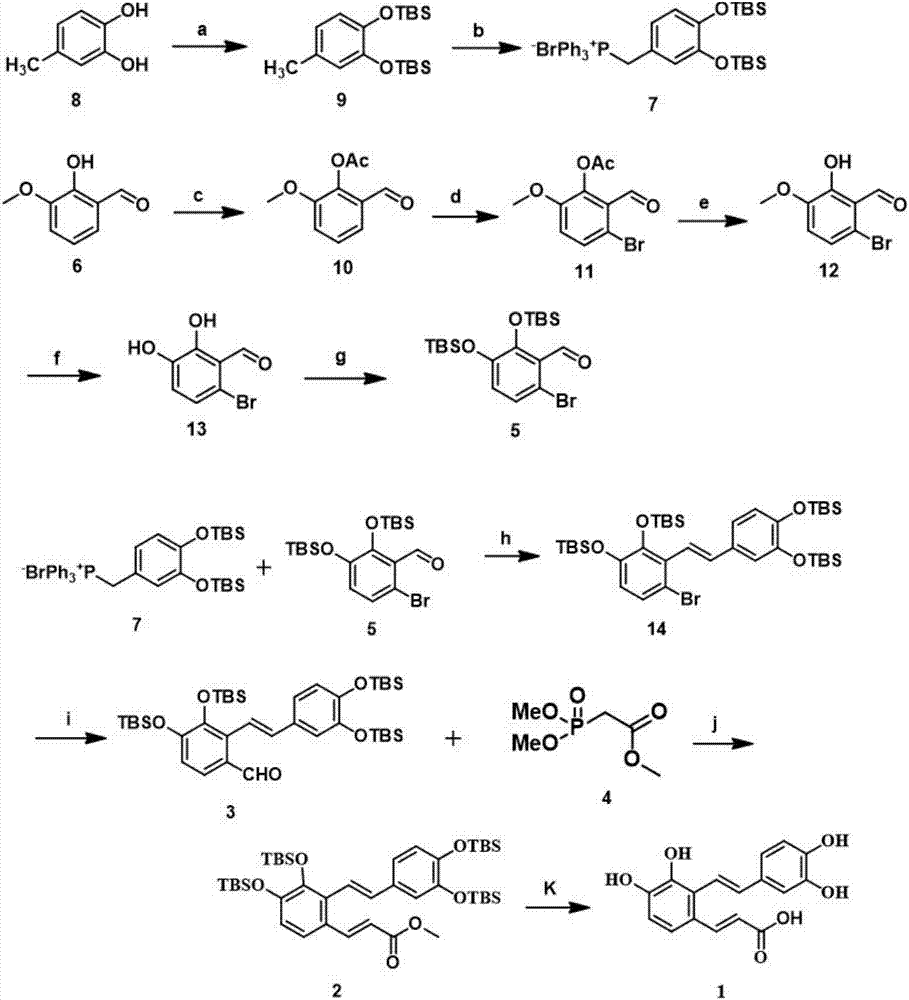
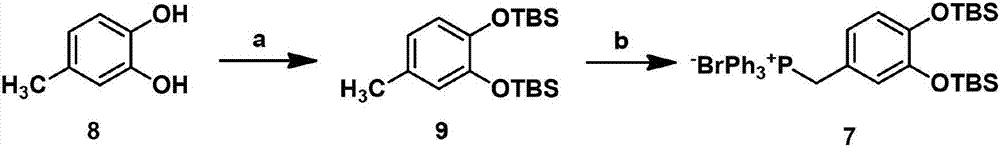
New synthesis method of natural product Salvianolic Acid F
A natural product and new synthesis technology, applied in chemical instruments and methods, compounds of elements of group 4/14 of the periodic table, compounds of elements of group 5/15 of the periodic table, etc., can solve the problem of low yield and difficult source of raw materials To achieve the effect of high yield, unique and novel design, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Using 4-methylcatechol, a compound of formula 8 as a raw material, first protected by a hydroxyl group, followed by a methyl radical reaction and then reacted with triphenylphosphine to obtain a phosphonium salt of formula 7:
[0028]
[0029] At 0°C, 4-methylcatechol (5.0g, 40.28mmol) was dissolved in N,N-dimethylformamide (DMF), and under nitrogen protection, imidazole (13.7g, 201.39mmol) and Tert-butyldimethylchlorosilane (TBSCl) (18.2 g, 120.83 mmol) was then raised to normal temperature and stirred overnight. Point the plate to monitor the reaction until it is complete. After that, the reaction was quenched with ice water, and then extracted with dichloromethane (3×150 mL). The organic phases were combined, washed with saturated NaCl solution, dried with anhydrous sodium sulfate, filtered, and evaporated under reduced pressure. After the solvent was removed, 14.20 g of the compound of formula 9 was obtained by separation through a chromatographic column, with a yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com