Heterocyclic matrine derivatives and preparation methods and applications thereof
A technology for matrine and derivatives, which is applied in the field of heterocyclic matrine derivatives and their preparation, can solve problems such as structural modification not yet carried out, and achieve the effect of obvious timeliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
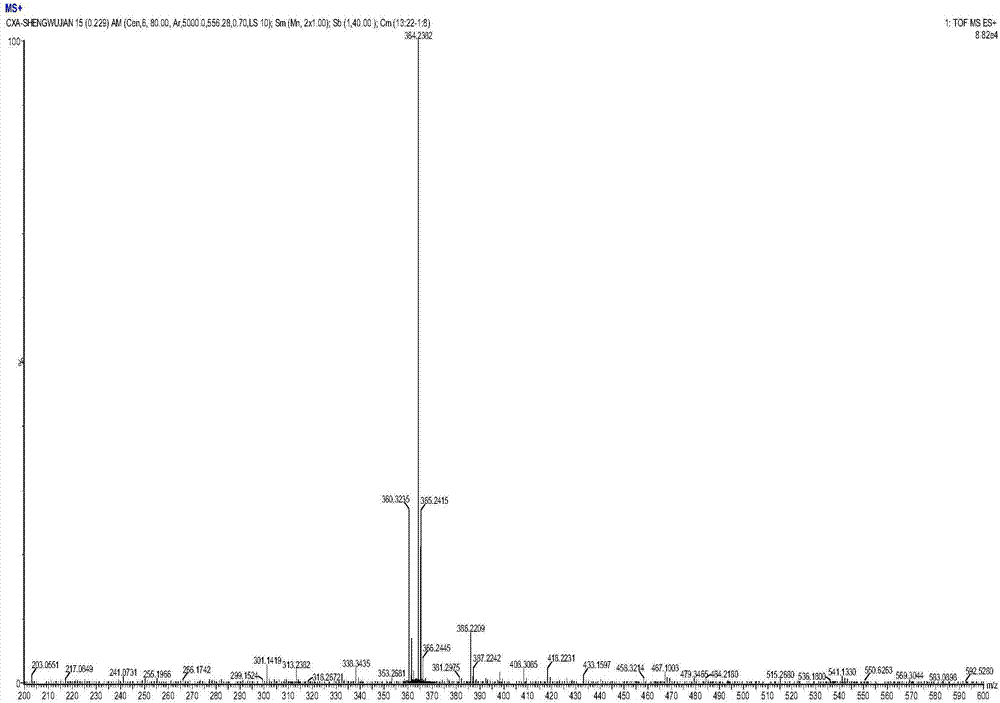
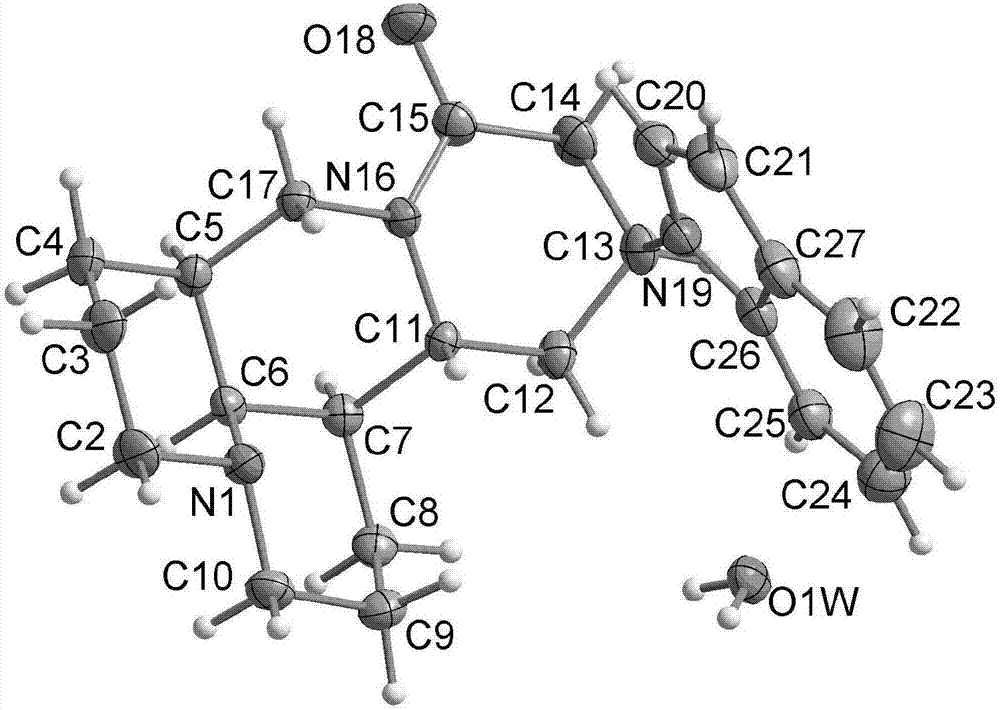
[0051] Embodiment 1 Indolylmatrine (Ind-Mat) preparation
[0052] S01. Preparation of crude product
[0053] 100mL three-neck flask, filled with N 2 After 5 minutes, 1.23 g of sophocarpine, 0.585 g of indole, 0.76 g of cesium fluoride and 12 mL of petroleum ether were added. Adjust N 2 Flow rate, start glycerin oil bath heating after 5 minutes, keep constant temperature 80°C, add 0.5mL tetramethoxysilane dropwise; stop heating after 6 hours of reaction. During the reaction process, the amount of solvent should be kept at 4-5mL, and 2-3mL can be added if it is insufficient. After the reaction, cool slightly, add 10mL of dichloromethane, stir and filter, evaporate the filtrate to get the crude product.
[0054] S02. Separation, purification and structural characterization
[0055] The crude product obtained in step S01 was separated and purified by silica gel column chromatography.
[0056] (1) Separation conditions
[0057] Stationary phase: 200-300 mesh silica gel 230mL...
Embodiment 2
[0067] Embodiment 2 cyclohexylaminomatrine (Cyc-Mat) preparation
[0068] S11. Preparation of crude product
[0069] Add 0.62g of sophocarpine, 0.55mL of cyclohexylamine, and 4mL of water into a 50mL single-necked flask, and heat in an oil bath at a constant temperature of 60°C for 6 hours under magnetic stirring. After the reaction, cool slightly, add 10mL of dichloromethane, stir and filter, evaporate the filtrate to get the crude product.
[0070] S12. Separation, purification and structural characterization
[0071] The crude product obtained in step S11 was subjected to silica gel column chromatography.
[0072] (1) Separation conditions
[0073] Stationary phase: 200-300 mesh silica gel 230mL;
[0074] Screen the elution system: TLC method screens the elution solvent system, and finally determines that the column chromatography separation mobile phase is ethyl acetate: ethanol = 1:8.
[0075] Column packing and activation: After the wet column packing is completed, ...
Embodiment 3
[0084] Embodiment 3 Bioactivity analysis of two derivatives of the present invention
[0085] 1. Experiment of the inhibitory activity of two derivatives on tumor Hela229 cells
[0086] (1) Experimental plan:
[0087] Test compound: lead compound matrine (Mat) before modification; synthetic experiment raw material, matrine derivative sophocarpine (Sop); two new matrine derivatives of the present invention indolylmatrine (derivative Substance II Ind-Mat) and cyclohexylaminomatrine (derivative III Cyc-Mat).
[0088] Cell culture: The human cervical cancer cells Hela229 to be tested were routinely cultured in a cell incubator with 5% carbon dioxide, 37°C and appropriate humidity with MEM basal medium plus 10% fetal bovine serum.
[0089] MTT detection of cell viability: select cells in good condition and adjust the cell suspension density to 8×10 4 ~1×10 5 cells / mL, inoculated into 96-well plates with 100 μL per well, and the effect of Mat and other drugs on cell proliferatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com