A kind of N-glucose hydrochloride base maleamic acid monomer, preparation method and application
A technology of glucosamine hydrochloride and maleamic acid, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., and can solve the problems of strict requirements on reaction temperature and equipment, complicated steps, and poor water solubility , to achieve the effect of simple and safe reaction conditions, high yield, and favorable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
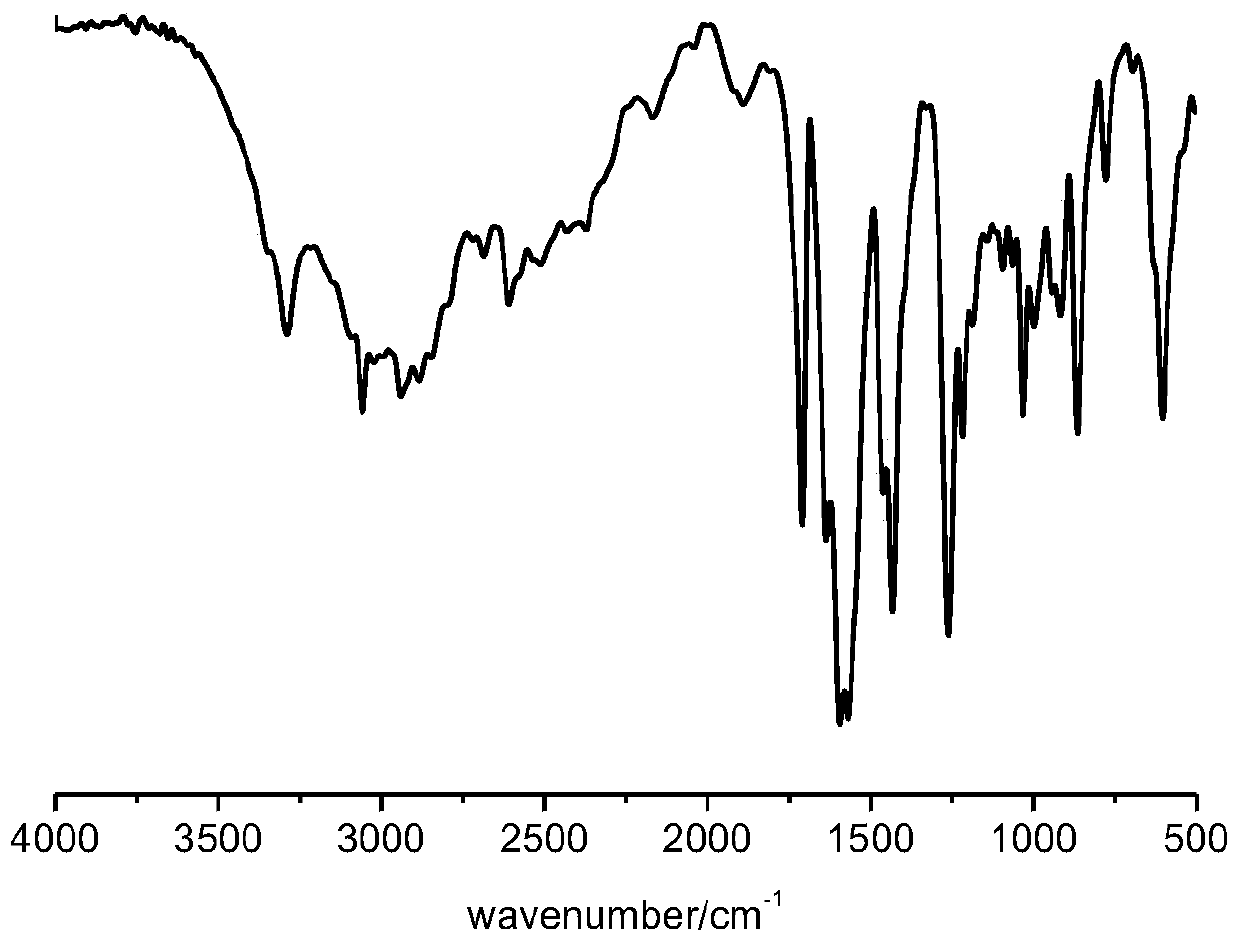
[0033] Add 1.4 mol of maleic anhydride to a dry three-necked round bottom flask, take 40 mL of glacial acetic acid to dissolve, slowly drip the remaining 40 mL of glacial acetic acid solution with 1.0 mol of glucosamine hydrochloride dissolved in it under stirring, and stir for 3 hours at 25°C. After the white powder is formed, suction filtration-washing-suction filtration and drying are used to obtain N-glucose hydrochloride maleamic acid. The yield is 77% by weighing. Its infrared absorption spectrum such as figure 1 As shown, the proton nuclear magnetic resonance spectrum is as Figure 4 Shown. The melting point of the product was 171°C.
Embodiment 2
[0035] Add 1.0 mol of maleic anhydride to a dry three-necked round bottom flask, take 20 mL of glacial acetic acid to dissolve, slowly drop the remaining 20 mL of glacial acetic acid solution with 1.0 mol of glucosamine hydrochloride dissolved in it under stirring, and stir at 50°C for 2 hours. After the white powder is formed, suction filtration-washing-suction filtration and drying are used to obtain N-glucose hydrochloride maleamic acid. The yield is 52% by weighing. The melting point of the product was 169°C.
Embodiment 3
[0037] Add 1.2 mol of maleic anhydride into a dry three-necked round bottom flask, take 60 mL of glacial acetic acid to dissolve, slowly drip the remaining 60 mL of glacial acetic acid solution with 1.0 mol of glucosamine hydrochloride dissolved in it under stirring, and stir for 6 hours at 30°C. After the white powder is formed, the N-glucose hydrochloride maleamic acid is obtained by suction filtration-washing-suction filtration and drying. The yield is 70% by weighing. The melting point of the product was 171°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com